Physiologically Relevant Oxygen Concentration (6% O2) as an Important Component of the Microenvironment Impacting Melanoma Phenotype and Melanoma Response to Targeted Therapeutics In Vitro
- PMID: 31461993
- PMCID: PMC6747123
- DOI: 10.3390/ijms20174203
Physiologically Relevant Oxygen Concentration (6% O2) as an Important Component of the Microenvironment Impacting Melanoma Phenotype and Melanoma Response to Targeted Therapeutics In Vitro
Abstract
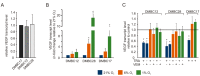
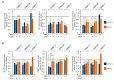
Cancer cell phenotype largely depends on oxygen availability. The atmospheric oxygen concentration (21%) used in in vitro studies is much higher than in any human tissue. Using well-characterized patient-derived melanoma cell lines, we compared: (i) activities of several signaling pathways, and (ii) the effects of vemurafenib and trametinib in hyperoxia (21% O2), normoxia (6% O2) and hypoxia (1% O2). A high plasticity of melanoma cells in response to changes in oxygen supplementation and drug treatment was observed, and the transcriptional reprograming and phenotypic changes varied between cell lines. Normoxia enhanced the expression of vascular endothelial growth factor (VEGF), glucose metabolism/transport-related genes, and changed percentages of NGFR- and MITF-positive cells in cell line-dependent manner. Increased protein stability might be responsible for high PGC1α level in MITFlow melanoma cells. Vemurafenib and trametinib while targeting the activity of MAPK/ERK pathway irrespective of oxygen concentration, were less effective in normoxia than hyperoxia in reducing levels of VEGF, PGC1α, SLC7A11 and Ki-67-positive cells in cell line-dependent manner. In conclusion, in vitro studies performed in atmospheric oxygen concentration provide different information on melanoma cell phenotype and response to drugs than performed in normoxia, which might partially explain the discrepancies between results obtained in vitro and in clinical settings.
Keywords: MITF; PGC1α; cancer heterogeneity; cellular metabolism; hypoxia; melanoma; normoxia; trametinib; vemurafenib.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Parthenolide induces MITF-M downregulation and senescence in patient-derived MITF-M(high) melanoma cell populations.Oncotarget. 2016 Feb 23;7(8):9026-40. doi: 10.18632/oncotarget.7030. Oncotarget. 2016. PMID: 26824319 Free PMC article.
-
Trametinib-Resistant Melanoma Cells Displaying MITFhigh/NGFRlow/IL-8low Phenotype Are Highly Responsive to Alternating Periods of Drug Withdrawal and Drug Rechallenge.Int J Mol Sci. 2023 Apr 26;24(9):7891. doi: 10.3390/ijms24097891. Int J Mol Sci. 2023. PMID: 37175614 Free PMC article.
-
Exogenous growth factors bFGF, EGF and HGF do not influence viability and phenotype of V600EBRAF melanoma cells and their response to vemurafenib and trametinib in vitro.PLoS One. 2017 Aug 22;12(8):e0183498. doi: 10.1371/journal.pone.0183498. eCollection 2017. PLoS One. 2017. PMID: 28829835 Free PMC article.
-
Allosteric MEK1/2 inhibitors including cobimetanib and trametinib in the treatment of cutaneous melanomas.Pharmacol Res. 2017 Mar;117:20-31. doi: 10.1016/j.phrs.2016.12.009. Epub 2016 Dec 9. Pharmacol Res. 2017. PMID: 27956260 Review.
-
Trametinib (GSK1120212).Recent Results Cancer Res. 2018;211:91-100. doi: 10.1007/978-3-319-91442-8_7. Recent Results Cancer Res. 2018. PMID: 30069762 Review.
Cited by
-
Impact of cell geometry, cellular uptake region, and tumour morphology on 225Ac and 177Lu dose distributions in prostate cancer.EJNMMI Phys. 2024 Nov 21;11(1):97. doi: 10.1186/s40658-024-00700-9. EJNMMI Phys. 2024. PMID: 39570450 Free PMC article.
-
Ferroptosis: From regulation of lipid peroxidation to the treatment of diseases.Cell Biol Toxicol. 2023 Jun;39(3):827-851. doi: 10.1007/s10565-022-09778-2. Epub 2022 Dec 2. Cell Biol Toxicol. 2023. PMID: 36459356 Review.
-
Non-Apoptotic Cell Death Signaling Pathways in Melanoma.Int J Mol Sci. 2020 Apr 23;21(8):2980. doi: 10.3390/ijms21082980. Int J Mol Sci. 2020. PMID: 32340261 Free PMC article. Review.
-
Molecular and Immune Biomarkers for Cutaneous Melanoma: Current Status and Future Prospects.Cancers (Basel). 2020 Nov 20;12(11):3456. doi: 10.3390/cancers12113456. Cancers (Basel). 2020. PMID: 33233603 Free PMC article. Review.
-
Ferroptosis as a promising therapeutic strategy for melanoma.Front Pharmacol. 2023 Sep 19;14:1252567. doi: 10.3389/fphar.2023.1252567. eCollection 2023. Front Pharmacol. 2023. PMID: 37795022 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

