Rats with a Human Mutation of NFU1 Develop Pulmonary Hypertension
- PMID: 31461310
- PMCID: PMC6993542
- DOI: 10.1165/rcmb.2019-0065OC
Rats with a Human Mutation of NFU1 Develop Pulmonary Hypertension
Abstract
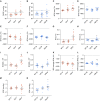
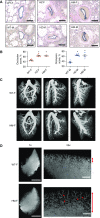
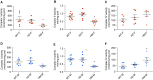
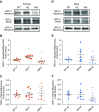
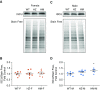
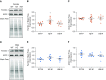
NFU1 is a mitochondrial protein that is involved in the biosynthesis of iron-sulfur clusters, and its genetic modification is associated with disorders of mitochondrial energy metabolism. Patients with autosomal-recessive inheritance of the NFU1 mutation G208C have reduced activity of the respiratory chain Complex II and decreased levels of lipoic-acid-dependent enzymes, and develop pulmonary arterial hypertension (PAH) in ∼70% of cases. We investigated whether rats with a human mutation in NFU1 are also predisposed to PAH development. A point mutation in rat NFU1G206C (human G208C) was introduced through CRISPR/Cas9 genome editing. Hemodynamic data, tissue samples, and fresh mitochondria were collected and analyzed. NFU1G206C rats showed increased right ventricular pressure, right ventricular hypertrophy, and high levels of pulmonary artery remodeling. Computed tomography and angiography of the pulmonary vasculature indicated severe angioobliterative changes in NFU1G206C rats. Importantly, the penetrance of the PAH phenotype was found to be more prevalent in females than in males, replicating the established sex difference among patients with PAH. Male and female homozygote rats exhibited decreased expression and activity of mitochondrial Complex II, and markedly decreased pyruvate dehydrogenase activity and lipoate binding. The limited development of PAH in males correlated with the preserved levels of oligomeric NFU1, increased expression of ISCU (an alternative branch of the iron-sulfur assembly system), and increased complex IV activity. Thus, the male sex has additional plasticity to overcome the iron-sulfur cluster deficiency. Our work describes a novel, humanized rat model of NFU1 deficiency that showed mitochondrial dysfunction similar to that observed in patients and developed PAH with the same sex dimorphism.
Keywords: Complex II; iron-sulfur cluster; mitochondrial dysfunction; pulmonary arterial hypertension; sex difference.
Figures


Comment in
-
NFU1, Iron-Sulfur Biogenesis, and Pulmonary Arterial Hypertension: A (Metabolic) Shift in Our Thinking.Am J Respir Cell Mol Biol. 2020 Feb;62(2):136-138. doi: 10.1165/rcmb.2019-0309ED. Am J Respir Cell Mol Biol. 2020. PMID: 31526282 Free PMC article. No abstract available.
Similar articles
-
Single Mutation in the NFU1 Gene Metabolically Reprograms Pulmonary Artery Smooth Muscle Cells.Arterioscler Thromb Vasc Biol. 2021 Feb;41(2):734-754. doi: 10.1161/ATVBAHA.120.314655. Epub 2020 Dec 10. Arterioscler Thromb Vasc Biol. 2021. PMID: 33297749 Free PMC article.
-
A fatal mitochondrial disease is associated with defective NFU1 function in the maturation of a subset of mitochondrial Fe-S proteins.Am J Hum Genet. 2011 Nov 11;89(5):656-67. doi: 10.1016/j.ajhg.2011.10.005. Am J Hum Genet. 2011. PMID: 22077971 Free PMC article.
-
Assembly of the [4Fe-4S] cluster of NFU1 requires the coordinated donation of two [2Fe-2S] clusters from the scaffold proteins, ISCU2 and ISCA1.Hum Mol Genet. 2020 Nov 25;29(19):3165-3182. doi: 10.1093/hmg/ddaa172. Hum Mol Genet. 2020. PMID: 32776106 Free PMC article.
-
Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer.Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H570-8. doi: 10.1152/ajpheart.01324.2007. Epub 2007 Dec 14. Am J Physiol Heart Circ Physiol. 2008. PMID: 18083891 Review.
-
NFU1 gene mutation and mitochondrial disorders.Neurol India. 2016 Jul-Aug;64(4):630-2. doi: 10.4103/0028-3886.185402. Neurol India. 2016. PMID: 27381105 Review.
Cited by
-
Advances in Genome Editing and Application to the Generation of Genetically Modified Rat Models.Front Genet. 2021 Apr 20;12:615491. doi: 10.3389/fgene.2021.615491. eCollection 2021. Front Genet. 2021. PMID: 33959146 Free PMC article. Review.
-
Metabolism, Mitochondrial Dysfunction, and Redox Homeostasis in Pulmonary Hypertension.Antioxidants (Basel). 2022 Feb 21;11(2):428. doi: 10.3390/antiox11020428. Antioxidants (Basel). 2022. PMID: 35204311 Free PMC article. Review.
-
Pulmonary Hypertension: A Contemporary Review.Am J Respir Crit Care Med. 2023 Sep 1;208(5):528-548. doi: 10.1164/rccm.202302-0327SO. Am J Respir Crit Care Med. 2023. PMID: 37450768 Free PMC article. Review.
-
A Review of Multiple Mitochondrial Dysfunction Syndromes, Syndromes Associated with Defective Fe-S Protein Maturation.Biomedicines. 2021 Aug 10;9(8):989. doi: 10.3390/biomedicines9080989. Biomedicines. 2021. PMID: 34440194 Free PMC article. Review.
-
Sex Dimorphism in Pulmonary Hypertension: The Role of the Sex Chromosomes.Antioxidants (Basel). 2021 May 14;10(5):779. doi: 10.3390/antiox10050779. Antioxidants (Basel). 2021. PMID: 34068984 Free PMC article. Review.
References
-
- Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. - PubMed
-
- Dromparis P, Paulin R, Sutendra G, Qi AC, Bonnet S, Michelakis ED. Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circ Res. 2013;113:126–136. - PubMed
-
- Paulin R, Dromparis P, Sutendra G, Gurtu V, Zervopoulos S, Bowers L, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014;20:827–839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

