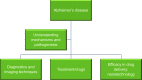
Alzheimer's disease: pathogenesis, diagnostics, and therapeutics
- PMID: 31410002
- PMCID: PMC6650620
- DOI: 10.2147/IJN.S200490
Alzheimer's disease: pathogenesis, diagnostics, and therapeutics
Abstract
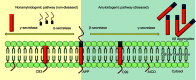
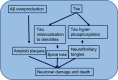
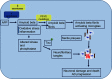
Currently, 47 million people live with dementia globally, and it is estimated to increase more than threefold (~131 million) by 2050. Alzheimer's disease (AD) is one of the major causative factors to induce progressive dementia. AD is a neurodegenerative disease, and its pathogenesis has been attributed to extracellular aggregates of amyloid β (Aβ) plaques and intracellular neurofibrillary tangles made of hyperphosphorylated τ-protein in cortical and limbic areas of the human brain. It is characterized by memory loss and progressive neurocognitive dysfunction. The anomalous processing of APP by β-secretases and γ-secretases leads to production of Aβ40 and Aβ42 monomers, which further oligomerize and aggregate into senile plaques. The disease also intensifies through infectious agents like HIV. Additionally, during disease pathogenesis, the presence of high concentrations of Aβ peptides in central nervous system initiates microglial infiltration. Upon coming into vicinity of Aβ, microglia get activated, endocytose Aβ, and contribute toward their clearance via TREM2 surface receptors, simultaneously triggering innate immunoresponse against the aggregation. In addition to a detailed report on causative factors leading to AD, the present review also discusses the current state of the art in AD therapeutics and diagnostics, including labeling and imaging techniques employed as contrast agents for better visualization and sensing of the plaques. The review also points to an urgent need for nanotechnology as an efficient therapeutic strategy to increase the bioavailability of drugs in the central nervous system.
Keywords: amyloid beta; amyloid precursor proteins; amyloidogenesis; tau phosphorylation; β-secretases; γ-secretases.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
[Alzheimer disease: cellular and molecular aspects].Bull Mem Acad R Med Belg. 2005;160(10-12):445-9; discussion 450-1. Bull Mem Acad R Med Belg. 2005. PMID: 16768248 French.
-
Trem2 Deletion Reduces Late-Stage Amyloid Plaque Accumulation, Elevates the Aβ42:Aβ40 Ratio, and Exacerbates Axonal Dystrophy and Dendritic Spine Loss in the PS2APP Alzheimer's Mouse Model.J Neurosci. 2020 Feb 26;40(9):1956-1974. doi: 10.1523/JNEUROSCI.1871-19.2019. Epub 2020 Jan 24. J Neurosci. 2020. PMID: 31980586 Free PMC article.
-
The fluorescent ligand bTVBT2 reveals increased p-tau uptake by retinal microglia in Alzheimer's disease patients and AppNL-F/NL-F mice.Alzheimers Res Ther. 2024 Jan 2;16(1):4. doi: 10.1186/s13195-023-01375-7. Alzheimers Res Ther. 2024. PMID: 38167557 Free PMC article.
-
Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?J Biol Chem. 2018 Oct 5;293(40):15419-15428. doi: 10.1074/jbc.R118.003999. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143530 Free PMC article. Review.
Cited by
-
QbD-based rivastigmine tartrate-loaded solid lipid nanoparticles for enhanced intranasal delivery to the brain for Alzheimer's therapeutics.Front Aging Neurosci. 2022 Aug 11;14:960246. doi: 10.3389/fnagi.2022.960246. eCollection 2022. Front Aging Neurosci. 2022. Retraction in: Front Aging Neurosci. 2024 Mar 28;16:1399271. doi: 10.3389/fnagi.2024.1399271. PMID: 36034142 Free PMC article. Retracted.
-
The emerging role of autophagy and mitophagy in tauopathies: From pathogenesis to translational implications in Alzheimer's disease.Front Aging Neurosci. 2022 Oct 17;14:1022821. doi: 10.3389/fnagi.2022.1022821. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36325189 Free PMC article. Review.
-
Single-Cell RNA Sequencing Reveals Immunomodulatory Effects of Stem Cell Factor and Granulocyte Colony-Stimulating Factor Treatment in the Brains of Aged APP/PS1 Mice.Biomolecules. 2024 Jul 10;14(7):827. doi: 10.3390/biom14070827. Biomolecules. 2024. PMID: 39062541 Free PMC article.
-
Codonopsis pilosula polysaccharide attenuates Aβ toxicity and cognitive defects in APP/PS1 mice.Aging (Albany NY). 2020 Jul 11;12(13):13422-13436. doi: 10.18632/aging.103445. Epub 2020 Jul 11. Aging (Albany NY). 2020. PMID: 32652518 Free PMC article.
-
House dust mite-induced asthma exacerbates Alzheimer's disease changes in the brain of the AppNL-G-F mouse model of disease.Brain Behav Immun. 2024 Oct;121:365-383. doi: 10.1016/j.bbi.2024.07.038. Epub 2024 Jul 29. Brain Behav Immun. 2024. PMID: 39084541 Free PMC article.
References
-
- Leandro P, Gomes CM. Protein misfolding in conformational disorders: rescue of folding defects and chemical chaperoning. Mini Rev Med Chem. 2008;8(9):901–911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

