Combined deficiency of RAB32 and RAB38 in the mouse mimics Hermansky-Pudlak syndrome and critically impairs thrombosis
- PMID: 31399401
- PMCID: PMC6693013
- DOI: 10.1182/bloodadvances.2019031286
Combined deficiency of RAB32 and RAB38 in the mouse mimics Hermansky-Pudlak syndrome and critically impairs thrombosis
Abstract
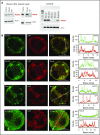
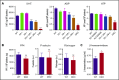
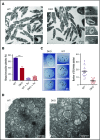
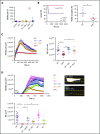
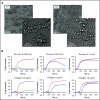
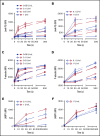
The biogenesis of lysosome related organelles is defective in Hermansky-Pudlak syndrome (HPS), a disorder characterized by oculocutaneous albinism and platelet dense granule (DG) defects. The first animal model of HPS was the fawn-hooded rat, harboring a spontaneous mutation inactivating the small guanosine triphosphatase Rab38 This leads to coat color dilution associated with the absence of DGs and lung morphological defects. Another RAB38 mutant, the cht mouse, has normal DGs, which has raised controversy about the role of RAB38 in DG biogenesis. We show here that murine and human, but not rat, platelets also express the closely related RAB32. To elucidate the parts played by RAB32 and RAB38 in the biogenesis of DGs in vivo and their effects on platelet functions, we generated mice inactivated for Rab32, Rab38, and both genes. Single Rab38 inactivation mimicked cht mice, whereas single Rab32 inactivation had no effect in DGs, coat color, or lung morphology. By contrast, Rab32/38 double inactivation mimicked severe HPS, with strong coat and eye pigment dilution, some enlarged lung multilamellar bodies associated with a decrease in the number of DGs. These organelles were morphologically abnormal, decreased in number, and devoid of 5-hydroxytryptamine content. In line with the storage pool defect, platelet activation was affected, resulting in severely impaired thrombus growth and prolongation of the bleeding time. Overall, our study demonstrates the absence of impact of RAB38 or RAB32 single deficiency in platelet biogenesis and function resulting from full redundancy, and characterized a new mouse model mimicking HPS devoid of DG content.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Similar articles
-
The rat Ruby ( R) locus is Rab38: identical mutations in Fawn-hooded and Tester-Moriyama rats derived from an ancestral Long Evans rat sub-strain.Mamm Genome. 2004 Apr;15(4):307-14. doi: 10.1007/s00335-004-2337-9. Mamm Genome. 2004. PMID: 15112108
-
Rab38 Mutation and the Lung Phenotype.Int J Mol Sci. 2018 Jul 27;19(8):2203. doi: 10.3390/ijms19082203. Int J Mol Sci. 2018. PMID: 30060521 Free PMC article. Review.
-
BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor.Curr Biol. 2012 Nov 20;22(22):2135-9. doi: 10.1016/j.cub.2012.09.020. Epub 2012 Oct 18. Curr Biol. 2012. PMID: 23084991 Free PMC article.
-
Analysis of ocular hypopigmentation in Rab38cht/cht mice.Invest Ophthalmol Vis Sci. 2007 Sep;48(9):3905-13. doi: 10.1167/iovs.06-1464. Invest Ophthalmol Vis Sci. 2007. PMID: 17724166 Free PMC article.
-
Hermansky-Pudlak syndrome subtype 5 (HPS-5) novel mutation in a 65 year-old with oculocutaneous hypopigmentation and mild bleeding diathesis: The importance of recognizing a subtle phenotype.Platelets. 2018 Jan;29(1):91-94. doi: 10.1080/09537104.2017.1361019. Epub 2017 Nov 1. Platelets. 2018. PMID: 29090612 Review.
Cited by
-
Platelet proteo-transcriptomic profiling validates mediators of thrombosis and proteostasis in patients with myeloproliferative neoplasms.bioRxiv [Preprint]. 2023 Oct 26:2023.10.23.563619. doi: 10.1101/2023.10.23.563619. bioRxiv. 2023. PMID: 37961700 Free PMC article. Preprint.
-
LRRK2 binds to the Rab32 subfamily in a GTP-dependent manner via its armadillo domain.Small GTPases. 2021 Mar;12(2):133-146. doi: 10.1080/21541248.2019.1666623. Epub 2019 Sep 25. Small GTPases. 2021. PMID: 31552791 Free PMC article.
-
Mitochondrial a Kinase Anchor Proteins in Cardiovascular Health and Disease: A Review Article on Behalf of the Working Group on Cellular and Molecular Biology of the Heart of the Italian Society of Cardiology.Int J Mol Sci. 2022 Jul 12;23(14):7691. doi: 10.3390/ijms23147691. Int J Mol Sci. 2022. PMID: 35887048 Free PMC article. Review.
-
Rab32/38-Dependent and -Independent Transport of Tyrosinase to Melanosomes in B16-F1 Melanoma Cells.Int J Mol Sci. 2022 Nov 16;23(22):14144. doi: 10.3390/ijms232214144. Int J Mol Sci. 2022. PMID: 36430618 Free PMC article.
-
Rab32 family proteins regulate autophagosomal components recycling.J Cell Biol. 2024 Mar 4;223(3):e202306040. doi: 10.1083/jcb.202306040. Epub 2024 Feb 7. J Cell Biol. 2024. PMID: 38323995 Free PMC article.
References
-
- Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12(5):261-273. - PubMed
-
- Holmsen H, Weiss HJ. Secretable storage pools in platelets. Annu Rev Med. 1979;30(1):119-134. - PubMed
-
- McNicol A, Israels SJ. Platelet dense granules: structure, function and implications for haemostasis. Thromb Res. 1999;95(1):1-18. - PubMed
-
- Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279(43):44250-44257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

