Hypothalamic mTORC2 is essential for metabolic health and longevity
- PMID: 31373126
- PMCID: PMC6718533
- DOI: 10.1111/acel.13014
Hypothalamic mTORC2 is essential for metabolic health and longevity
Abstract
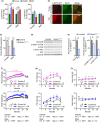
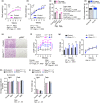
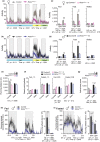
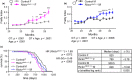
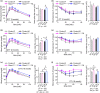
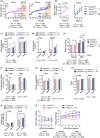
The mechanistic target of rapamycin (mTOR) is an evolutionarily conserved protein kinase that regulates growth and metabolism. mTOR is found in two protein complexes, mTORC1 and mTORC2, that have distinct components and substrates and are both inhibited by rapamycin, a macrolide drug that robustly extends lifespan in multiple species including worms and mice. Although the beneficial effect of rapamycin on longevity is generally attributed to reduced mTORC1 signaling, disruption of mTORC2 signaling can also influence the longevity of worms, either positively or negatively depending on the temperature and food source. Here, we show that loss of hypothalamic mTORC2 signaling in mice decreases activity level, increases the set point for adiposity, and renders the animals susceptible to diet-induced obesity. Hypothalamic mTORC2 signaling normally increases with age, and mice lacking this pathway display higher fat mass and impaired glucose homeostasis throughout life, become more frail with age, and have decreased overall survival. We conclude that hypothalamic mTORC2 is essential for the normal metabolic health, fitness, and lifespan of mice. Our results have implications for the use of mTORC2-inhibiting pharmaceuticals in the treatment of brain cancer and diseases of aging.
Keywords: frailty; hypothalamus; lifespanobesity; mTOR; mTORC2; obesity.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
D.W.L has received funding from and is a scientific advisory board member of, Aeonian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases.
Figures
Similar articles
-
Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan.Aging Cell. 2014 Oct;13(5):911-7. doi: 10.1111/acel.12256. Epub 2014 Jul 25. Aging Cell. 2014. PMID: 25059582 Free PMC article.
-
Effects of rapamycin on growth hormone receptor knockout mice.Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1495-E1503. doi: 10.1073/pnas.1717065115. Epub 2018 Jan 29. Proc Natl Acad Sci U S A. 2018. PMID: 29378959 Free PMC article.
-
Calorie-Restriction-Induced Insulin Sensitivity Is Mediated by Adipose mTORC2 and Not Required for Lifespan Extension.Cell Rep. 2019 Oct 1;29(1):236-248.e3. doi: 10.1016/j.celrep.2019.08.084. Cell Rep. 2019. PMID: 31577953 Free PMC article.
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
-
Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects.Adv Biol Regul. 2017 May;64:39-48. doi: 10.1016/j.jbior.2016.12.001. Epub 2017 Jan 4. Adv Biol Regul. 2017. PMID: 28189457 Review.
Cited by
-
Regulation of metabolic health by dietary histidine in mice.J Physiol. 2023 Jun;601(11):2139-2163. doi: 10.1113/JP283261. Epub 2022 Sep 27. J Physiol. 2023. PMID: 36086823 Free PMC article.
-
Reduced insulin signaling in neurons induces sex-specific health benefits.Sci Adv. 2023 Feb 22;9(8):eade8137. doi: 10.1126/sciadv.ade8137. Epub 2023 Feb 22. Sci Adv. 2023. PMID: 36812323 Free PMC article.
-
The mTOR-lysosome axis at the centre of ageing.FEBS Open Bio. 2022 Apr;12(4):739-757. doi: 10.1002/2211-5463.13347. Epub 2021 Dec 18. FEBS Open Bio. 2022. PMID: 34878722 Free PMC article. Review.
-
A toolbox for the longitudinal assessment of healthspan in aging mice.Nat Protoc. 2020 Feb;15(2):540-574. doi: 10.1038/s41596-019-0256-1. Epub 2020 Jan 8. Nat Protoc. 2020. PMID: 31915391 Free PMC article.
-
Blazing a trail for the clinical use of rapamycin as a geroprotecTOR.Geroscience. 2023 Oct;45(5):2769-2783. doi: 10.1007/s11357-023-00935-x. Epub 2023 Oct 6. Geroscience. 2023. PMID: 37801202 Free PMC article.
References
-
- Alhadeff, A. L. , & Grill, H. J. (2014). Hindbrain nucleus tractus solitarius glucagon‐like peptide‐1 receptor signaling reduces appetitive and motivational aspects of feeding. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 307(4), R465–R470. 10.1152/ajpregu.00179.2014+ - DOI - PMC - PubMed
-
- Apelo, S. I. A. , Pumper, C. P. , Baar, E. L. , Cummings, N. E. , & Lamming, D. W. (2016). Intermittent administration of rapamycin extends the life span of female C57BL/6J mice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 71(7), 876–881. 10.1093/gerona/glw064 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

