Late-stage Anle138b treatment ameliorates tau pathology and metabolic decline in a mouse model of human Alzheimer's disease tau
- PMID: 31370885
- PMCID: PMC6670231
- DOI: 10.1186/s13195-019-0522-z
Late-stage Anle138b treatment ameliorates tau pathology and metabolic decline in a mouse model of human Alzheimer's disease tau
Abstract
Background: Augmenting the brain clearance of toxic oligomers with small molecule modulators constitutes a promising therapeutic concept against tau deposition. However, there has been no test of this concept in animal models of Alzheimer's disease (AD) with initiation at a late disease stage. Thus, we aimed to investigate the effects of interventional late-stage Anle138b treatment, which previously indicated great potential to inhibit oligomer accumulation by binding of pathological aggregates, on the metabolic decline in transgenic mice with established tauopathy in a longitudinal 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) study.
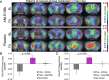
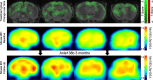
Methods: Twelve transgenic mice expressing all six human tau isoforms (hTau) and ten controls were imaged by FDG-PET at baseline (14.5 months), followed by randomization into Anle138b treatment and vehicle groups for 3 months. FDG-PET was repeated after treatment for 3 months, and brains were analyzed by tau immunohistochemistry. Longitudinal changes of glucose metabolism were compared between study groups, and the end point tau load was correlated with individual FDG-PET findings.
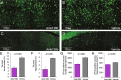
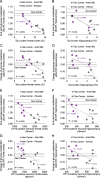
Results: Tau pathology was significantly ameliorated by late-stage Anle138b treatment when compared to vehicle (frontal cortex - 53%, p < 0.001; hippocampus - 59%, p < 0.005). FDG-PET revealed a reversal of metabolic decline during Anle138b treatment, whereas the vehicle group showed ongoing deterioration. End point glucose metabolism in the brain of hTau mice had a strong correlation with tau deposition measured by immunohistochemistry (R = 0.92, p < 0.001).
Conclusion: Late-stage oligomer modulation effectively ameliorated tau pathology in hTau mice and rescued metabolic function. Molecular imaging by FDG-PET can serve for monitoring effects of Anle138b treatment.
Keywords: Anle138b; Late-stage; Neuronal injury; Small animal PET; Tau.
Conflict of interest statement
SR, AL, AG, and CG are inventors in a patent application related to the compound use in this study. AG and CG are co-founders of MODAG. AL is partly employed by MODAG. All other authors declare that they have no competing interests.
Figures
Similar articles
-
Mechanism of action deconvolution of the small-molecule pathological tau aggregation inhibitor Anle138b.Alzheimers Res Ther. 2023 Mar 14;15(1):52. doi: 10.1186/s13195-023-01182-0. Alzheimers Res Ther. 2023. PMID: 36918909 Free PMC article.
-
Reducing tau aggregates with anle138b delays disease progression in a mouse model of tauopathies.Acta Neuropathol. 2015 Nov;130(5):619-31. doi: 10.1007/s00401-015-1483-3. Epub 2015 Oct 6. Acta Neuropathol. 2015. PMID: 26439832 Free PMC article.
-
Prodromal neuroinflammatory, cholinergic and metabolite dysfunction detected by PET and MRS in the TgF344-AD transgenic rat model of AD: a collaborative multi-modal study.Theranostics. 2021 May 3;11(14):6644-6667. doi: 10.7150/thno.56059. eCollection 2021. Theranostics. 2021. PMID: 34093845 Free PMC article.
-
In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings.Ageing Res Rev. 2017 Jul;36:50-63. doi: 10.1016/j.arr.2017.03.002. Epub 2017 Mar 15. Ageing Res Rev. 2017. PMID: 28315409 Review.
-
[Development of SPECT Probes for In Vivo Imaging of β-Amyloid and Tau Aggregates in the Alzheimer's Disease Brain].Yakugaku Zasshi. 2017;137(11):1361-1365. doi: 10.1248/yakushi.17-00156. Yakugaku Zasshi. 2017. PMID: 29093372 Review. Japanese.
Cited by
-
Mechanism of action deconvolution of the small-molecule pathological tau aggregation inhibitor Anle138b.Alzheimers Res Ther. 2023 Mar 14;15(1):52. doi: 10.1186/s13195-023-01182-0. Alzheimers Res Ther. 2023. PMID: 36918909 Free PMC article.
-
Non-invasive imaging of tau-targeted probe uptake by whole brain multi-spectral optoacoustic tomography.Eur J Nucl Med Mol Imaging. 2022 Jun;49(7):2137-2152. doi: 10.1007/s00259-022-05708-w. Epub 2022 Feb 7. Eur J Nucl Med Mol Imaging. 2022. PMID: 35128565 Free PMC article.
-
A seeding-based neuronal model of tau aggregation for use in drug discovery.PLoS One. 2023 Apr 4;18(4):e0283941. doi: 10.1371/journal.pone.0283941. eCollection 2023. PLoS One. 2023. PMID: 37014877 Free PMC article.
-
Constant Levels of Tau Phosphorylation in the Brain of htau Mice.Front Mol Neurosci. 2020 Aug 28;13:136. doi: 10.3389/fnmol.2020.00136. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32982685 Free PMC article.
-
Feasibility of short imaging protocols for [18F]PI-2620 tau-PET in progressive supranuclear palsy.Eur J Nucl Med Mol Imaging. 2021 Nov;48(12):3872-3885. doi: 10.1007/s00259-021-05391-3. Epub 2021 May 22. Eur J Nucl Med Mol Imaging. 2021. PMID: 34021393 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

