PTEN Tumor-Suppressor: The Dam of Stemness in Cancer
- PMID: 31366089
- PMCID: PMC6721423
- DOI: 10.3390/cancers11081076
PTEN Tumor-Suppressor: The Dam of Stemness in Cancer
Abstract
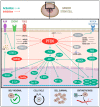
PTEN is one of the most frequently inactivated tumor suppressor genes in cancer. Loss or variation in PTEN gene/protein levels is commonly observed in a broad spectrum of human cancers, while germline PTEN mutations cause inherited syndromes that lead to increased risk of tumors. PTEN restrains tumorigenesis through different mechanisms ranging from phosphatase-dependent and independent activities, subcellular localization and protein interaction, modulating a broad array of cellular functions including growth, proliferation, survival, DNA repair, and cell motility. The main target of PTEN phosphatase activity is one of the most significant cell growth and pro-survival signaling pathway in cancer: PI3K/AKT/mTOR. Several shreds of evidence shed light on the critical role of PTEN in normal and cancer stem cells (CSCs) homeostasis, with its loss fostering the CSC compartment in both solid and hematologic malignancies. CSCs are responsible for tumor propagation, metastatic spread, resistance to therapy, and relapse. Thus, understanding how alterations of PTEN levels affect CSC hallmarks could be crucial for the development of successful therapeutic approaches. Here, we discuss the most significant findings on PTEN-mediated control of CSC state. We aim to unravel the role of PTEN in the regulation of key mechanisms specific for CSCs, such as self-renewal, quiescence/cell cycle, Epithelial-to-Mesenchymal-Transition (EMT), with a particular focus on PTEN-based therapy resistance mechanisms and their exploitation for novel therapeutic approaches in cancer treatment.
Keywords: PTEN; cancer stem cells; targeted therapy; therapy resistance.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting.Adv Biol Regul. 2014 Sep;56:66-80. doi: 10.1016/j.jbior.2014.07.002. Epub 2014 Jul 19. Adv Biol Regul. 2014. PMID: 25088603 Review.
-
miR-10b expression in breast cancer stem cells supports self-renewal through negative PTEN regulation and sustained AKT activation.EMBO Rep. 2016 May;17(5):648-58. doi: 10.15252/embr.201540678. Epub 2016 Apr 9. EMBO Rep. 2016. PMID: 27113763 Free PMC article.
-
The Role of PTEN in Epithelial-Mesenchymal Transition.Cancers (Basel). 2022 Aug 3;14(15):3786. doi: 10.3390/cancers14153786. Cancers (Basel). 2022. PMID: 35954450 Free PMC article. Review.
-
Methods for PTEN in Stem Cells and Cancer Stem Cells.Methods Mol Biol. 2016;1388:233-85. doi: 10.1007/978-1-4939-3299-3_15. Methods Mol Biol. 2016. PMID: 27033080
-
Aryl hydrocarbon receptor/cytochrome P450 1A1 pathway mediates breast cancer stem cells expansion through PTEN inhibition and β-Catenin and Akt activation.Mol Cancer. 2017 Jan 19;16(1):14. doi: 10.1186/s12943-016-0570-y. Mol Cancer. 2017. PMID: 28103884 Free PMC article.
Cited by
-
Up-regulation of KISS1 as a novel target of Let-7i in melanoma serves as a potential suppressor of migration and proliferation in vitro.J Cell Mol Med. 2021 Jul;25(14):6864-6873. doi: 10.1111/jcmm.16695. Epub 2021 Jun 6. J Cell Mol Med. 2021. PMID: 34096173 Free PMC article.
-
Exosomes: A novel insight into traditional Chinese medicine.Front Pharmacol. 2022 Aug 29;13:844782. doi: 10.3389/fphar.2022.844782. eCollection 2022. Front Pharmacol. 2022. PMID: 36105201 Free PMC article. Review.
-
Decoding the regulatory landscape of lncRNAs as potential diagnostic and prognostic biomarkers for gastric and colorectal cancers.Clin Exp Med. 2024 Jan 31;24(1):29. doi: 10.1007/s10238-023-01260-5. Clin Exp Med. 2024. PMID: 38294554 Free PMC article. Review.
-
Interfering with the Ubiquitin-Mediated Regulation of Akt as a Strategy for Cancer Treatment.Int J Mol Sci. 2023 Feb 1;24(3):2809. doi: 10.3390/ijms24032809. Int J Mol Sci. 2023. PMID: 36769122 Free PMC article. Review.
-
A novel PTEN mutant caused by polymorphism in cis-regulatory elements is involved in chemosensitivity in breast cancer.Am J Cancer Res. 2023 Jan 15;13(1):86-104. eCollection 2023. Am J Cancer Res. 2023. PMID: 36777516 Free PMC article.
References
-
- Li D.M., Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res. 1997;57:2124–2129. - PubMed
-
- Steck P.A., Pershouse M.A., Jasser S.A., Yung W.K., Lin H., Ligon A.H., Langford L.A., Baumgard M.L., Hattier T., Davis T., et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

