Cryo-EM structure of TRPC5 at 2.8-Å resolution reveals unique and conserved structural elements essential for channel function
- PMID: 31355338
- PMCID: PMC6656536
- DOI: 10.1126/sciadv.aaw7935
Cryo-EM structure of TRPC5 at 2.8-Å resolution reveals unique and conserved structural elements essential for channel function
Abstract
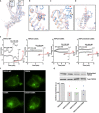
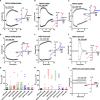
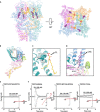
The transient receptor potential canonical subfamily member 5 (TRPC5), one of seven mammalian TRPC members, is a nonselective calcium-permeant cation channel. TRPC5 is of considerable interest as a drug target in the treatment of progressive kidney disease, depression, and anxiety. Here, we present the 2.8-Å resolution cryo-electron microscopy (cryo-EM) structure of the mouse TRPC5 (mTRPC5) homotetramer. Comparison of the TRPC5 structure to previously determined structures of other TRPC and TRP channels reveals differences in the extracellular pore domain and in the length of the S3 helix. The disulfide bond at the extracellular side of the pore and a preceding small loop are essential elements for its proper function. This high-resolution structure of mTRPC5, combined with electrophysiology and mutagenesis, provides insight into the lipid modulation and gating mechanisms of the TRPC family of ion channels.
Figures



Similar articles
-
Structure of the human lipid-gated cation channel TRPC3.Elife. 2018 May 4;7:e36852. doi: 10.7554/eLife.36852. Elife. 2018. PMID: 29726814 Free PMC article.
-
Expression and Purification of the Human Lipid-sensitive Cation Channel TRPC3 for Structural Determination by Single-particle Cryo-electron Microscopy.J Vis Exp. 2019 Jan 7;(143). doi: 10.3791/58754. J Vis Exp. 2019. PMID: 30663716
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
Structure of the mouse TRPC4 ion channel.Nat Commun. 2018 Aug 6;9(1):3102. doi: 10.1038/s41467-018-05247-9. Nat Commun. 2018. PMID: 30082700 Free PMC article.
-
Structure-Function Relationship and Physiological Roles of Transient Receptor Potential Canonical (TRPC) 4 and 5 Channels.Cells. 2019 Dec 27;9(1):73. doi: 10.3390/cells9010073. Cells. 2019. PMID: 31892199 Free PMC article. Review.
Cited by
-
Hydrophobic interactions within the C terminus pole helices tunnel regulate calcium-dependent inactivation of TRPC3 in a calmodulin-dependent manner.Cell Calcium. 2023 Jan;109:102684. doi: 10.1016/j.ceca.2022.102684. Epub 2022 Nov 30. Cell Calcium. 2023. PMID: 36495796 Free PMC article.
-
Druggable Lipid Binding Sites in Pentameric Ligand-Gated Ion Channels and Transient Receptor Potential Channels.Front Physiol. 2022 Jan 4;12:798102. doi: 10.3389/fphys.2021.798102. eCollection 2021. Front Physiol. 2022. PMID: 35069257 Free PMC article. Review.
-
TRP channels in health and disease at a glance.J Cell Sci. 2021 Jul 1;134(13):jcs258372. doi: 10.1242/jcs.258372. Epub 2021 Jul 13. J Cell Sci. 2021. PMID: 34254641 Free PMC article.
-
Molecular architecture of the Gαi-bound TRPC5 ion channel.Nat Commun. 2023 May 3;14(1):2550. doi: 10.1038/s41467-023-38281-3. Nat Commun. 2023. PMID: 37137991 Free PMC article.
-
Human TRPC5 structures reveal interaction of a xanthine-based TRPC1/4/5 inhibitor with a conserved lipid binding site.Commun Biol. 2020 Nov 23;3(1):704. doi: 10.1038/s42003-020-01437-8. Commun Biol. 2020. PMID: 33230284 Free PMC article.
References
-
- Clapham D. E., TRP channels as cellular sensors. Nature 426, 517–524 (2003). - PubMed
-
- Schaefer M., Plant T. D., Obukhov A. G., Hofmann T., Gudermann T., Schultz G., Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 275, 17517–17526 (2000). - PubMed
-
- Strübing C., Krapivinsky G., Krapivinsky L., Clapham D. E., TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29, 645–655 (2001). - PubMed
-
- Strübing C., Krapivinsky G., Krapivinsky L., Clapham D. E., Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 278, 39014–39019 (2003). - PubMed
-
- Albert A. P., Gating mechanisms of canonical transient receptor potential channel proteins: Role of phosphoinositols and diacylglycerol. Adv. Exp. Med. Biol. 704, 391–411 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

