The Neonatal Fc Receptor (FcRn): A Misnomer?
- PMID: 31354709
- PMCID: PMC6636548
- DOI: 10.3389/fimmu.2019.01540
The Neonatal Fc Receptor (FcRn): A Misnomer?
Abstract
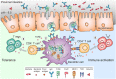
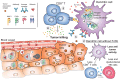
Antibodies are essential components of an adaptive immune response. Immunoglobulin G (IgG) is the most common type of antibody found in circulation and extracellular fluids. Although IgG alone can directly protect the body from infection through the activities of its antigen binding region, the majority of IgG immune functions are mediated via proteins and receptors expressed by specialized cell subsets that bind to the fragment crystallizable (Fc) region of IgG. Fc gamma (γ) receptors (FcγR) belong to a broad family of proteins that presently include classical membrane-bound surface receptors as well as atypical intracellular receptors and cytoplasmic glycoproteins. Among the atypical FcγRs, the neonatal Fc receptor (FcRn) has increasingly gained notoriety given its intimate influence on IgG biology and its ability to also bind to albumin. FcRn functions as a recycling or transcytosis receptor that is responsible for maintaining IgG and albumin in the circulation, and bidirectionally transporting these two ligands across polarized cellular barriers. More recently, it has been appreciated that FcRn acts as an immune receptor by interacting with and facilitating antigen presentation of peptides derived from IgG immune complexes (IC). Here we review FcRn biology and focus on newer advances including how emerging FcRn-targeted therapies may affect the immune responses to IgG and IgG IC.
Keywords: FcRn; IgG; IgG immune complex (IgG-IC); albumin (ALB); immunity; therapeutic.
Figures


Similar articles
-
Evidence that FcRn mediates the transplacental passage of maternal IgE in the form of IgG anti-IgE/IgE immune complexes.Clin Exp Allergy. 2015 Jun;45(6):1085-98. doi: 10.1111/cea.12508. Clin Exp Allergy. 2015. PMID: 25652137 Free PMC article.
-
The immunologic functions of the neonatal Fc receptor for IgG.J Clin Immunol. 2013 Jan;33 Suppl 1(Suppl 1):S9-17. doi: 10.1007/s10875-012-9768-y. Epub 2012 Sep 5. J Clin Immunol. 2013. PMID: 22948741 Free PMC article. Review.
-
The neonatal Fc receptor in mucosal immune regulation.Scand J Immunol. 2021 Feb;93(2):e13017. doi: 10.1111/sji.13017. Epub 2021 Jan 17. Scand J Immunol. 2021. PMID: 33351196 Review.
-
FcRn, but not FcγRs, drives maternal-fetal transplacental transport of human IgG antibodies.Proc Natl Acad Sci U S A. 2020 Jun 9;117(23):12943-12951. doi: 10.1073/pnas.2004325117. Epub 2020 May 27. Proc Natl Acad Sci U S A. 2020. PMID: 32461366 Free PMC article.
-
The multiple facets of FcRn in immunity.Immunol Rev. 2015 Nov;268(1):253-68. doi: 10.1111/imr.12331. Immunol Rev. 2015. PMID: 26497526 Review.
Cited by
-
Sialylated immunoglobulin G: a promising diagnostic and therapeutic strategy for autoimmune diseases.Theranostics. 2021 Mar 13;11(11):5430-5446. doi: 10.7150/thno.53961. eCollection 2021. Theranostics. 2021. PMID: 33859756 Free PMC article. Review.
-
Entry and Disposition of Zika Virus Immune Complexes in a Tissue Culture Model of the Maternal-Fetal Interface.Vaccines (Basel). 2021 Feb 11;9(2):145. doi: 10.3390/vaccines9020145. Vaccines (Basel). 2021. PMID: 33670199 Free PMC article.
-
B cells defined by immunoglobulin isotypes.Clin Exp Immunol. 2022 Dec 31;210(3):230-239. doi: 10.1093/cei/uxac091. Clin Exp Immunol. 2022. PMID: 36197112 Free PMC article.
-
The Role of Nucleoprotein in Immunity to Human Negative-Stranded RNA Viruses-Not Just Another Brick in the Viral Nucleocapsid.Viruses. 2022 Mar 3;14(3):521. doi: 10.3390/v14030521. Viruses. 2022. PMID: 35336928 Free PMC article. Review.
-
Binding and neutralizing anti-AAV antibodies: Detection and implications for rAAV-mediated gene therapy.Mol Ther. 2023 Mar 1;31(3):616-630. doi: 10.1016/j.ymthe.2023.01.010. Epub 2023 Jan 11. Mol Ther. 2023. PMID: 36635967 Free PMC article. Review.
References
-
- F.Brambell WR. The passive immunity of the young mammal. Biol Rev. (1958) 33:488–531. 10.1111/j.1469-185X.1958.tb01412.x - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

