Cell membrane camouflaged nanoparticles: a new biomimetic platform for cancer photothermal therapy
- PMID: 31354269
- PMCID: PMC6588714
- DOI: 10.2147/IJN.S200284
Cell membrane camouflaged nanoparticles: a new biomimetic platform for cancer photothermal therapy
Abstract
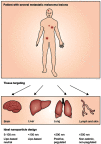
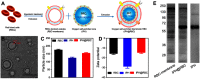
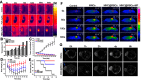
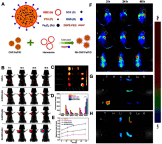
Targeted drug delivery by nanoparticles (NPs) is an essential technique to achieve the ideal therapeutic effect for cancer. However, it requires large amounts of work to imitate the biomarkers on the surface of the cell membrane and cannot fully retain the bio-function and interactions among cells. Cell membranes have been studied to form biomimetic NPs to achieve functions like immune escape, targeted drug delivery, and immune modulation, which inherit the ability to interact with the in vivo environments. Currently, erythrocyte, leukocyte, mesenchymal stem cell, cancer cell and platelet have been applied in coating photothermal agents and anti-cancer drugs to achieve increased photothermal conversion efficiency and decreased side effects in cancer ablation. In this review, we discuss the recent development of cell membrane-coated NPs in the application of photothermal therapy and cancer targeting. The underlying biomarkers of cell membrane-coated nanoparticles (CMNPs) are discussed, and future research directions are suggested.
Keywords: cancer targeting; cell membrane; nanoparticles; photothermal therapy.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Enhanced photothermal therapy of biomimetic polypyrrole nanoparticles through improving blood flow perfusion.Biomaterials. 2017 Oct;143:130-141. doi: 10.1016/j.biomaterials.2017.08.004. Epub 2017 Aug 5. Biomaterials. 2017. PMID: 28800434
-
Recent Advances in Cell Membrane-Camouflaged Nanoparticles for Cancer Phototherapy.Small. 2019 Jan;15(1):e1804105. doi: 10.1002/smll.201804105. Epub 2018 Nov 20. Small. 2019. PMID: 30457701 Review.
-
Oxygen self-enriched nanoparticles functionalized with erythrocyte membranes for long circulation and enhanced phototherapy.Acta Biomater. 2017 Sep 1;59:269-282. doi: 10.1016/j.actbio.2017.06.035. Epub 2017 Jun 27. Acta Biomater. 2017. PMID: 28663143
-
Light/pH-Triggered Biomimetic Red Blood Cell Membranes Camouflaged Small Molecular Drug Assemblies for Imaging-Guided Combinational Chemo-Photothermal Therapy.ACS Appl Mater Interfaces. 2019 May 1;11(17):15262-15275. doi: 10.1021/acsami.9b00897. Epub 2019 Apr 17. ACS Appl Mater Interfaces. 2019. PMID: 30964624
-
Biomembrane camouflaged nanoparticles: A paradigm shifts in targeted drug delivery system.Colloids Surf B Biointerfaces. 2024 Jun;238:113893. doi: 10.1016/j.colsurfb.2024.113893. Epub 2024 Apr 4. Colloids Surf B Biointerfaces. 2024. PMID: 38631282 Review.
Cited by
-
Cell membrane-coated nanoparticles: a novel multifunctional biomimetic drug delivery system.Drug Deliv Transl Res. 2023 Mar;13(3):716-737. doi: 10.1007/s13346-022-01252-0. Epub 2022 Nov 22. Drug Deliv Transl Res. 2023. PMID: 36417162 Free PMC article. Review.
-
Extracellular vesicle-based drug delivery systems for cancer treatment.Theranostics. 2019 Oct 17;9(26):8001-8017. doi: 10.7150/thno.37097. eCollection 2019. Theranostics. 2019. PMID: 31754377 Free PMC article. Review.
-
Doxorubicin Delivered Using Nanoparticles Camouflaged with Mesenchymal Stem Cell Membranes to Treat Colon Cancer.Int J Nanomedicine. 2020 Apr 23;15:2873-2884. doi: 10.2147/IJN.S242787. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32368059 Free PMC article.
-
Therapeutic lipid-coated hybrid nanoparticles against bacterial infections.RSC Adv. 2020 Feb 27;10(14):8497-8517. doi: 10.1039/c9ra10921h. eCollection 2020 Feb 24. RSC Adv. 2020. PMID: 35497832 Free PMC article. Review.
-
Cell-derived biomimetic nanocarriers for targeted cancer therapy: cell membranes and extracellular vesicles.Drug Deliv. 2021 Dec;28(1):1237-1255. doi: 10.1080/10717544.2021.1938757. Drug Deliv. 2021. PMID: 34142930 Free PMC article. Review.
References
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agents Smancs. Cancer Res. 1986;46:1986. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

