The emerging role of human cytomegalovirus infection in human carcinogenesis: a review of current evidence and potential therapeutic implications
- PMID: 31303966
- PMCID: PMC6611507
- DOI: 10.18632/oncotarget.27016
The emerging role of human cytomegalovirus infection in human carcinogenesis: a review of current evidence and potential therapeutic implications
Abstract
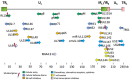
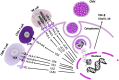
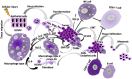
It is well-established that infections with viruses harboring oncogenic potential increase the cancer risk. Virus induced oncogenic processes are influenced by a complex and unique combination of host and environmental risk factors that are currently not fully understood. Many of the oncogenic viruses exhibit a prolonged, asymptomatic latency after a primary infection, and cause cancer in only a minority of carriers. From an epidemiologic point of view, it is therefore difficult to determine their role in cancer development. However, recent evidence suggests a neoplastic potential of one additional ubiquitous virus; human Cytomegalovirus (HCMV). Emerging data presents HCMV as a plausible cancer-causing virus by demonstrating its presence in >90% of common tumor types, while being absent in normal tissue surrounding the tumor. HCMV targets many cell types in tumor tissues, and can cause all the ten proposed hallmarks of cancer. This virus exhibits cellular tumor-promoting and immune-evasive strategies, hijacks proangiogenic and anti-apoptotic mechanisms and induces immunosuppressive effects in the tumor micro-environment. Recognizing new cancer-causing mechanisms may increase the therapeutic potential and prophylactic options for virus associated cancer forms. Such approaches could limit viral spread, and promote anti-viral and immune controlling strategies if given as add on to standard therapy to potentially improve the prognosis of cancer patients. This review will focus on HCMV-related onco-viral mechanisms and the potential of HCMV as a new therapeutic target in HCMV positive cancer forms.
Keywords: HCMV; cancer; glioblastoma; human cytomegalovirus; oncovirus.
Conflict of interest statement
CONFLICTS OF INTEREST Vetvik and Geisler have nothing to disclose. Söderberg-Nauclér holds a patent to diagnose and treat an HCMV strain that is highly associated with cancer.
Figures

Similar articles
-
Tumors and Cytomegalovirus: An Intimate Interplay.Viruses. 2022 Apr 14;14(4):812. doi: 10.3390/v14040812. Viruses. 2022. PMID: 35458542 Free PMC article. Review.
-
Expression of Oncogenic Alleles Induces Multiple Blocks to Human Cytomegalovirus Infection.J Virol. 2016 Apr 14;90(9):4346-4356. doi: 10.1128/JVI.00179-16. Print 2016 May. J Virol. 2016. PMID: 26889030 Free PMC article.
-
A Review of the Potential Role of Human Cytomegalovirus (HCMV) Infections in Breast Cancer Carcinogenesis and Abnormal Immunity.Cancers (Basel). 2019 Nov 22;11(12):1842. doi: 10.3390/cancers11121842. Cancers (Basel). 2019. PMID: 31766600 Free PMC article. Review.
-
Modulatory effects of human cytomegalovirus infection on malignant properties of cancer cells.Intervirology. 1996;39(4):259-69. doi: 10.1159/000150527. Intervirology. 1996. PMID: 9078467 Review.
-
The Human Cytomegalovirus, from Oncomodulation to Oncogenesis.Viruses. 2018 Aug 3;10(8):408. doi: 10.3390/v10080408. Viruses. 2018. PMID: 30081496 Free PMC article. Review.
Cited by
-
Human cytomegalovirus infection enhances 5‑lipoxygenase and cycloxygenase‑2 expression in colorectal cancer.Int J Oncol. 2023 Nov;63(5):116. doi: 10.3892/ijo.2023.5564. Epub 2023 Sep 1. Int J Oncol. 2023. PMID: 37654195 Free PMC article.
-
Hybrid-Capture Target Enrichment in Human Pathogens: Identification, Evolution, Biosurveillance, and Genomic Epidemiology.Pathogens. 2024 Mar 23;13(4):275. doi: 10.3390/pathogens13040275. Pathogens. 2024. PMID: 38668230 Free PMC article. Review.
-
Tumors and Cytomegalovirus: An Intimate Interplay.Viruses. 2022 Apr 14;14(4):812. doi: 10.3390/v14040812. Viruses. 2022. PMID: 35458542 Free PMC article. Review.
-
Evolution and Genetic Diversity of Primate Cytomegaloviruses.Microorganisms. 2020 Apr 25;8(5):624. doi: 10.3390/microorganisms8050624. Microorganisms. 2020. PMID: 32344906 Free PMC article. Review.
-
Correspondence Between Cytomegalovirus Immunoglobulin-G Levels Measured in Saliva and Serum.Front Immunol. 2020 Aug 28;11:2095. doi: 10.3389/fimmu.2020.02095. eCollection 2020. Front Immunol. 2020. PMID: 32983163 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

