Enhanced bone tissue regeneration of a biomimetic cellular scaffold with co-cultured MSCs-derived osteogenic and angiogenic cells
- PMID: 31297910
- PMCID: PMC6797511
- DOI: 10.1111/cpr.12658
Enhanced bone tissue regeneration of a biomimetic cellular scaffold with co-cultured MSCs-derived osteogenic and angiogenic cells
Abstract
Objectives: The bone tissue engineering primarily focuses on three-dimensional co-culture systems, which physical and biological properties resemble the cell matrix of actual tissues. The complex dialogue between bone-forming and endothelial cells (ECs) in a tissue-engineered construct will directly regulate angiogenesis and bone regeneration. The purpose of this study was to investigate whether co-culture between osteogenic and angiogenic cells derived by bone mesenchymal stem cells (MSCs) could affect cell activities and new bone formation.
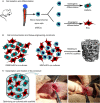
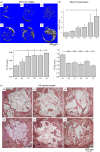
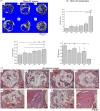
Materials and methods: Mesenchymal stem cells were dually induced to differentiate into osteogenic cells (OMSCs) and ECs; both cell types were co-cultured at different ratios to investigate their effects and underlying mechanisms through ELISA, RT-qPCR and MTT assays. The selected cell mixture was transplanted onto a nano-hydroxyapatite/polyurethane (n-HA/PU) scaffold to form a cell-scaffold construct that was implanted in the rat femoral condyles. Histology and micro-CT were examined for further verification.
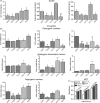
Results: ELISA and gene expression studies revealed that co-cultured OMSCs/ECs (0.5/1.5) significantly elevated the transcription levels of osteogenic genes such as ALP, Col-I and OCN, as well as transcription factors Msx2, Runx2 and Osterix; it also upregulated angiogenic factors of vascular endothelial growth factor (VEGF) and CD31 when compared with cells cultured alone or in other ratios. The optimized OMSCs/ECs group had more abundant calcium phosphate crystal deposition, further facilitated their bone formation in vivo.
Conclusions: The OMSCs/ECs-scaffold constructs at an optimal cell ratio (0.5/1.5) achieved enhanced osteogenic and angiogenic factor expression and biomineralization, which resulted in more effective bone formation.
Keywords: angiogenic cells; biomimetic scaffold; bone tissue engineering; co-culture; osteogenesis; stem cells.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Osteogenic differentiation and bone regeneration of iPSC-MSCs supported by a biomimetic nanofibrous scaffold.Acta Biomater. 2016 Jan;29:365-379. doi: 10.1016/j.actbio.2015.10.007. Epub 2015 Oct 9. Acta Biomater. 2016. PMID: 26441129
-
Sema3A and HIF1α co-overexpressed iPSC-MSCs/HA scaffold facilitates the repair of calvarial defect in a mouse model.J Cell Physiol. 2020 Oct;235(10):6754-6766. doi: 10.1002/jcp.29569. Epub 2020 Feb 3. J Cell Physiol. 2020. PMID: 32012286
-
A novel method to improve the osteogenesis capacity of hUCMSCs with dual-directional pre-induction under screened co-culture conditions.Cell Prolif. 2020 Feb;53(2):e12740. doi: 10.1111/cpr.12740. Epub 2019 Dec 9. Cell Prolif. 2020. PMID: 31820506 Free PMC article.
-
Periosteum and development of the tissue-engineered periosteum for guided bone regeneration.J Orthop Translat. 2022 Feb 16;33:41-54. doi: 10.1016/j.jot.2022.01.002. eCollection 2022 Mar. J Orthop Translat. 2022. PMID: 35228996 Free PMC article. Review.
-
Coupling Osteogenesis and Vasculogenesis in Engineered Orthopedic Tissues.Tissue Eng Part B Rev. 2021 Jun;27(3):199-214. doi: 10.1089/ten.TEB.2020.0132. Epub 2020 Sep 25. Tissue Eng Part B Rev. 2021. PMID: 32854589 Free PMC article. Review.
Cited by
-
An injectable pH neutral bioactive glass-based bone cement with suitable bone regeneration ability.J Orthop Translat. 2022 Sep 2;36:120-131. doi: 10.1016/j.jot.2022.05.011. eCollection 2022 Sep. J Orthop Translat. 2022. PMID: 36128442 Free PMC article.
-
In vivo bone regeneration assessment of offset and gradient melt electrowritten (MEW) PCL scaffolds.Biomater Res. 2020 Oct 1;24:17. doi: 10.1186/s40824-020-00196-1. eCollection 2020. Biomater Res. 2020. PMID: 33014414 Free PMC article.
-
miR-129-5p Promotes Osteogenic Differentiation of BMSCs and Bone Regeneration via Repressing Dkk3.Stem Cells Int. 2021 Jul 15;2021:7435605. doi: 10.1155/2021/7435605. eCollection 2021. Stem Cells Int. 2021. PMID: 34326879 Free PMC article.
-
MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells.Int J Mol Sci. 2021 Feb 27;22(5):2362. doi: 10.3390/ijms22052362. Int J Mol Sci. 2021. PMID: 33673409 Free PMC article. Review.
-
Towards Stem Cell Therapy for Critical-Sized Segmental Bone Defects: Current Trends and Challenges on the Path to Clinical Translation.J Funct Biomater. 2024 May 27;15(6):145. doi: 10.3390/jfb15060145. J Funct Biomater. 2024. PMID: 38921519 Free PMC article. Review.
References
-
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post‐natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873‐884. - PubMed
-
- Lü LX, Deegan A, Musa F, Xu T, Yang Y. The effects of biomimetically conjugated VEGF on osteogenesis and angiogenesis of MSCs (human and rat) and HUVECs co‐culture models. Colloid Surface B. 2018;167:550‐559. - PubMed
-
- Yang CC, Han B, Cao CL, Yang D, Qu XZ, Wang XY. An injectable double‐network hydrogel for the co‐culture of vascular endothelial cells and bone marrow mesenchymal stem cells for simultaneously enhancing vascularization and osteogenesis. J Mater Chem B. 2018;6:7811. - PubMed
-
- Grellier M, Bordenave L, Amedee J. Cell‐to‐cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol. 2009;27:562‐571. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

