What is new about mild temperature sensing? A review of recent findings
- PMID: 31286024
- PMCID: PMC6601417
- DOI: 10.1080/23328940.2019.1607490
What is new about mild temperature sensing? A review of recent findings
Abstract
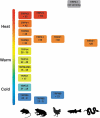
The superfamily of Transient Receptor Potential (TRP) channels is composed by a group of calcium-permeable ionic channels with a generally shared topology. The thermoTRP channels are a subgroup of 11 members, found in the TRPA, TRPV, TRPC, and TRPM subfamilies. Historically, members of this subgroup have been classified as cold, warm or hot-specific temperature sensors. Recently, new experimental results have shown that the role that has been given to the thermoTRPs in thermosensation is not necessarily strict. In addition, it has been shown that these channels activate over temperature ranges, which can have variations depending on the species and the interaction with a specific biological context. Investigation of these interactions could help to elucidate the mechanisms of activation by temperature, which remains uncertain. Abbreviations: Cryo-EM: Cryogenic electron microscopy; DRG: Dorsal root ganglia; H: Human; ROS: Reactive Oxygen Species; TG: Trigeminal ganglia; TRP: Transient Receptor Potential; TRPA: TRP ankyrin; TRPV: TRP vanilloid; TRPC: TRP canonical; TRPM: TRP melastatin.
Keywords: TRP channels; TRPA1; TRPM8; TRPV1; ThermoTRP; enthalpy; temperature sensing.
Figures

Similar articles
-
TRP Channels and Pain.In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 8. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 8. PMID: 29356491 Free Books & Documents. Review.
-
Neuronal and non-neuronal TRPA1 as therapeutic targets for pain and headache relief.Temperature (Austin). 2022 May 29;10(1):50-66. doi: 10.1080/23328940.2022.2075218. eCollection 2023. Temperature (Austin). 2022. PMID: 37187829 Free PMC article. Review.
-
TRP Channels: What Do They Look Like?In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 1. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 1. PMID: 29356490 Free Books & Documents. Review.
-
Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors.J Comp Neurol. 2005 Dec 26;493(4):596-606. doi: 10.1002/cne.20794. J Comp Neurol. 2005. PMID: 16304633
-
Gating of thermally activated channels.Curr Top Membr. 2014;74:51-87. doi: 10.1016/B978-0-12-800181-3.00003-8. Curr Top Membr. 2014. PMID: 25366233 Review.
Cited by
-
Energy expenditure during physical work in cold environments: physiology and performance considerations for military service members.J Appl Physiol (1985). 2024 Oct 1;137(4):995-1013. doi: 10.1152/japplphysiol.00210.2024. Epub 2024 Aug 29. J Appl Physiol (1985). 2024. PMID: 39205639 Free PMC article. Review.
-
Combining Molecular Dynamics and Docking Simulations to Develop Targeted Protocols for Performing Optimized Virtual Screening Campaigns on The hTRPM8 Channel.Int J Mol Sci. 2020 Mar 25;21(7):2265. doi: 10.3390/ijms21072265. Int J Mol Sci. 2020. PMID: 32218173 Free PMC article.
-
TRP (transient receptor potential) ion channel family: structures, biological functions and therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Jul 5;8(1):261. doi: 10.1038/s41392-023-01464-x. Signal Transduct Target Ther. 2023. PMID: 37402746 Free PMC article. Review.
-
A molecular perspective on identifying TRPV1 thermosensitive regions and disentangling polymodal activation.Temperature (Austin). 2021 Oct 26;10(1):67-101. doi: 10.1080/23328940.2021.1983354. eCollection 2023. Temperature (Austin). 2021. PMID: 37187836 Free PMC article. Review.
-
Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells.Biomolecules. 2023 Jun 13;13(6):983. doi: 10.3390/biom13060983. Biomolecules. 2023. PMID: 37371563 Free PMC article. Review.
References
-
- Clapham DE, Runnels LW, Strübing C.. The TRP ion channel family. Nat Rev Neurosci. 2001;2(6):387–396. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
