A Combination of Oxo-M and 4-PPBP as a potential regenerative therapeutics for tendon injury
- PMID: 31281545
- PMCID: PMC6592164
- DOI: 10.7150/thno.35285
A Combination of Oxo-M and 4-PPBP as a potential regenerative therapeutics for tendon injury
Abstract
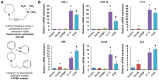
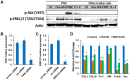
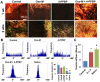
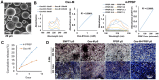
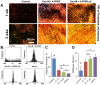
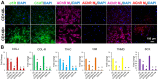
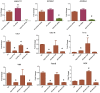
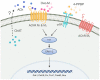
Tendons injuries frequently result in scar-like tissue with poor biochemical structure and mechanical properties. We have recently reported that CD146+ perivascular originated tendon stem/progenitor cells (TSCs), playing critical roles in tendon healing. Here, we identified highly efficient small molecules that selectively activate endogenous TSCs for tendon regeneration. Methods: From a pool of ERK1/2 and FAK agonists, Oxo-M and 4-PPBP were identified, and their roles in tenogenic differentiation of TSCs and in vivo tendon healing were investigated. Controlled delivery of Oxo-M and 4-PPBP was applied via PLGA µS. Signaling studies were conducted to determine the mechanism for specificity of Oxo-M and 4-PPBP to CD146+ TSCs. Results: A combination of Oxo-M and 4-PPBP synergistically increased the expressions of tendon-related gene markers in TSCs. In vivo, delivery of Oxo-M and 4-PPBP significantly enhanced healing of fully transected rat patellar tendons (PT), with functional restoration and reorganization of collagen fibrous structure. Our signaling study suggested that Oxo-M and 4-PPBP specifically targets CD146+ TSCs via non-neuronal muscarinic acetylcholine receptors (AChR) and σ1 receptor (σ1) signaling. Principal conclusions: Our findings demonstrate a significant potential of Oxo-M and 4-PPBP as a regenerative therapeutics for tendon injuries.
Keywords: Endogenous stem/progenitor cells; Regenerative Medicine; Small molecules; Tendon.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Oxo-M and 4-PPBP Delivery via Multi-Domain Peptide Hydrogel Toward Tendon Regeneration.Front Bioeng Biotechnol. 2022 Jan 27;10:773004. doi: 10.3389/fbioe.2022.773004. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35155388 Free PMC article.
-
Harnessing endogenous stem/progenitor cells for tendon regeneration.J Clin Invest. 2015 Jul 1;125(7):2690-701. doi: 10.1172/JCI81589. Epub 2015 Jun 8. J Clin Invest. 2015. PMID: 26053662 Free PMC article.
-
Tendon stem/progenitor cells regulate inflammation in tendon healing via JNK and STAT3 signaling.FASEB J. 2017 Sep;31(9):3991-3998. doi: 10.1096/fj.201700071R. Epub 2017 May 22. FASEB J. 2017. PMID: 28533328 Free PMC article.
-
Progress with stem cell therapies for tendon tissue regeneration.Expert Opin Biol Ther. 2020 Nov;20(11):1373-1379. doi: 10.1080/14712598.2020.1786532. Epub 2020 Jun 29. Expert Opin Biol Ther. 2020. PMID: 32574078 Review.
-
Assessment of stem cell carriers for tendon tissue engineering in pre-clinical models.Stem Cell Res Ther. 2014;5(2):38. doi: 10.1186/scrt426. Stem Cell Res Ther. 2014. PMID: 25157898 Free PMC article. Review.
Cited by
-
Effect of local anesthetics on viability and differentiation of various adult stem/progenitor cells.Stem Cell Res Ther. 2020 Sep 7;11(1):385. doi: 10.1186/s13287-020-01905-2. Stem Cell Res Ther. 2020. PMID: 32894184 Free PMC article.
-
The combination of BMP12 and KY02111 enhances tendon differentiation in bone marrow-derived equine mesenchymal stromal cells (BM-eMSCs).J Equine Sci. 2022 Jul;33(2):19-26. doi: 10.1294/jes.33.19. Epub 2022 Jul 6. J Equine Sci. 2022. PMID: 35847484 Free PMC article.
-
Physical and Soluble Cues Enhance Tendon Progenitor Cell Invasion into Injectable Synthetic Hydrogels.Adv Funct Mater. 2022 Nov 24;32(48):2207556. doi: 10.1002/adfm.202207556. Epub 2022 Sep 28. Adv Funct Mater. 2022. PMID: 39257859 Free PMC article.
-
Characterization of Tendon-Derived Stem Cells and Rescue Tendon Injury.Stem Cell Rev Rep. 2021 Oct;17(5):1534-1551. doi: 10.1007/s12015-021-10143-9. Epub 2021 Mar 2. Stem Cell Rev Rep. 2021. PMID: 33651334 Review.
-
The use of connective tissue growth factor mimics for flexor tendon repair.J Orthop Res. 2022 Dec;40(12):2754-2762. doi: 10.1002/jor.25301. Epub 2022 Feb 25. J Orthop Res. 2022. PMID: 35212415 Free PMC article.
References
-
- Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials. 2011;32:3189–209. - PubMed
-
- Miller FD, Kaplan DR. Mobilizing endogenous stem cells for repair and regeneration: are we there yet? Cell Stem Cell. 2012;10:650–2. - PubMed
-
- Woo SL, Jia F, Zou L, Gabriel MT. Functional tissue engineering for ligament healing: potential of antisense gene therapy. Ann Biomed Eng. 2004;32:342–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

