Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma
- PMID: 31245809
- PMCID: PMC7350555
- DOI: 10.1093/carcin/bgz124
Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma
Abstract
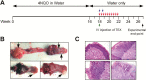
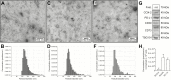
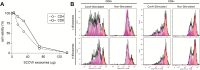
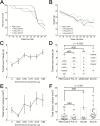
Circulating tumor-derived exosomes (TEX) interact with a variety of cells in cancer-bearing hosts, leading to cellular reprogramming which promotes disease progression. To study TEX effects on the development of solid tumors, immunosuppressive exosomes carrying PD-L1 and FasL were isolated from supernatants of murine or human HNSCC cell lines. TEX were delivered (IV) to immunocompetent C57BL/6 mice bearing premalignant oral/esophageal lesions induced by the carcinogen, 4-nitroquinoline 1-oxide (4NQO). Progression of the premalignant oropharyngeal lesions to malignant tumors was monitored. A single TEX injection increased the number of developing tumors (6.2 versus 3.2 in control mice injected with phosphate-buffered saline; P < 0.0002) and overall tumor burden per mouse (P < 0.037). The numbers of CD4+ and CD8+ T lymphocytes infiltrating the developing tumors were coordinately reduced (P < 0.01) in mice injected with SCCVII-derived TEX relative to controls. Notably, TEX isolated from mouse or human tumors had similar effects on tumor development and immune cells. A single IV injection of TEX was sufficient to condition mice harboring premalignant OSCC lesions for accelerated tumor progression in concert with reduced immune cell migration to the tumor.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Oral-specific ablation of Klf4 disrupts epithelial terminal differentiation and increases premalignant lesions and carcinomas upon chemical carcinogenesis.J Oral Pathol Med. 2015 Nov;44(10):801-9. doi: 10.1111/jop.12307. Epub 2015 Jan 21. J Oral Pathol Med. 2015. PMID: 25605610
-
Targeting of CD40 and PD-L1 Pathways Inhibits Progression of Oral Premalignant Lesions in a Carcinogen-induced Model of Oral Squamous Cell Carcinoma.Cancer Prev Res (Phila). 2021 Mar;14(3):313-324. doi: 10.1158/1940-6207.CAPR-20-0418. Epub 2020 Dec 4. Cancer Prev Res (Phila). 2021. PMID: 33277316
-
Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice.Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):301-13. doi: 10.1158/1078-0432.ccr-0999-3. Clin Cancer Res. 2004. PMID: 14734483
-
4-nitroquinoline-1-oxide induced experimental oral carcinogenesis.Oral Oncol. 2006 Aug;42(7):655-67. doi: 10.1016/j.oraloncology.2005.10.013. Epub 2006 Jan 30. Oral Oncol. 2006. PMID: 16448841 Review.
-
The 4-NQO mouse model: An update on a well-established in vivo model of oral carcinogenesis.Methods Cell Biol. 2021;163:197-229. doi: 10.1016/bs.mcb.2020.09.004. Epub 2020 Nov 12. Methods Cell Biol. 2021. PMID: 33785166 Review.
Cited by
-
Exosomal PDL1 Suppresses the Anticancer Activity of CD8+ T Cells in Hepatocellular Carcinoma.Anal Cell Pathol (Amst). 2024 Oct 9;2024:1608582. doi: 10.1155/2024/1608582. eCollection 2024. Anal Cell Pathol (Amst). 2024. PMID: 39421264 Free PMC article.
-
Immunosuppressive functions of melanoma cell-derived exosomes in plasma of melanoma patients.Front Cell Dev Biol. 2023 Jan 6;10:1080925. doi: 10.3389/fcell.2022.1080925. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684448 Free PMC article. Review.
-
Exosomes in head and neck cancer. Updating and revisiting.J Enzyme Inhib Med Chem. 2019 Dec;34(1):1641-1651. doi: 10.1080/14756366.2019.1662000. J Enzyme Inhib Med Chem. 2019. PMID: 31496355 Free PMC article. Review.
-
Tumor-Derived Exosomes and the Role of Liquid Biopsy in Human Papillomavirus Oropharyngeal Squamous Cell Carcinoma.Cancer J. 2023 Jul-Aug 01;29(4):230-237. doi: 10.1097/PPO.0000000000000671. Cancer J. 2023. PMID: 37471614 Free PMC article. Review.
-
Tumor-Derived Exosomes: Hidden Players in PD-1/PD-L1 Resistance.Cancers (Basel). 2021 Sep 10;13(18):4537. doi: 10.3390/cancers13184537. Cancers (Basel). 2021. PMID: 34572764 Free PMC article. Review.
References
-
- Dasgupta S., et al. (2005) Inhibition of NK cell activity through TGF-beta 1 by down-regulation of NKG2D in a murine model of head and neck cancer. J. Immunol., 175, 5541–5550. - PubMed
-
- Ferris R.L., et al. (2006) Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin. Cancer Res., 12, 3890–3895. - PubMed
-
- Mooradian M.J., et al. (2017) Immunomodulatory effects of current cancer treatment and the consequences for follow-up immunotherapeutics. Future Oncol., 13, 1649–1663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

