Involvement of Vasopressin in the Pathogenesis of Pulmonary Tuberculosis: A New Therapeutic Target?
- PMID: 31244771
- PMCID: PMC6563385
- DOI: 10.3389/fendo.2019.00351
Involvement of Vasopressin in the Pathogenesis of Pulmonary Tuberculosis: A New Therapeutic Target?
Abstract
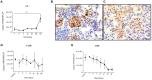
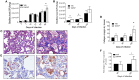
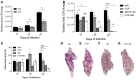
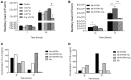
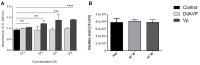
Tuberculosis (TB) is a highly complex infectious disease caused by the intracellular pathogen Mycobacterium tuberculosis (Mtb). It is characterized by chronic granulomatous inflammation of the lung and systemic immune-neuroendocrine responses that have been associated with pathophysiology and disease outcome. Vasopressin (VP), a neurohypophysial hormone with immunomodulatory effects, is abnormally high in plasma of some patients with pulmonary TB, and is apparently produced ectopically. In this study, a BALB/c mouse model of progressive pulmonary TB was used to determine whether VP may play a role in TB pathophysiology. Our results show that VP gene is expressed in the lung since early infection, increasing as the infection progressed, and localized mainly in macrophages, which are key cells in mycobacterial elimination. Pharmacologic manipulation using agonist and antagonist compounds showed that high and sustained stimulation of VPR resulted in increased bacillary burdens and fibrosis at lungs, while blockade of VP receptors reduced bacterial loads. Accordingly, treatment of infected alveolar macrophages with VP in cell cultures resulted in high numbers of intracellular Mtb and impaired cytokine production. Thus, we show that VP is ectopically produced in the tuberculous lungs, with macrophages being its most possible target cell. Further, it seems that chronic vasopressinergic stimulation during active late disease causes anti-inflammatory and tissue reparative effects, which could be deleterious while its pharmacologic suppression reactivates protective immunity and contributes to shorten conventional chemotherapy, which could be a new possible form of immune-endocrine therapy.
Keywords: fibrosis; immunopathology; lung; therapy; tuberculosis; vasopressin.
Figures
Similar articles
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
Mycobacterium tuberculosis Infection and Innate Responses in a New Model of Lung Alveolar Macrophages.Front Immunol. 2018 Mar 12;9:438. doi: 10.3389/fimmu.2018.00438. eCollection 2018. Front Immunol. 2018. PMID: 29593716 Free PMC article.
-
[Recent progress in mycobacteriology].Kekkaku. 2007 Oct;82(10):783-99. Kekkaku. 2007. PMID: 18018602 Japanese.
-
Striking the right immunological balance prevents progression of tuberculosis.Inflamm Res. 2017 Dec;66(12):1031-1056. doi: 10.1007/s00011-017-1081-z. Epub 2017 Jul 15. Inflamm Res. 2017. PMID: 28711989 Review.
Cited by
-
Associations of hyponatremia and SIADH with increased mortality, young age and infection parameters in patients with tuberculosis.PLoS One. 2022 Oct 13;17(10):e0275827. doi: 10.1371/journal.pone.0275827. eCollection 2022. PLoS One. 2022. PMID: 36227934 Free PMC article.
-
Arginine vasopressin and pathophysiology of COVID-19: An innovative perspective.Biomed Pharmacother. 2021 Nov;143:112193. doi: 10.1016/j.biopha.2021.112193. Epub 2021 Sep 15. Biomed Pharmacother. 2021. PMID: 34543987 Free PMC article. Review.
-
The Cholinergic System Contributes to the Immunopathological Progression of Experimental Pulmonary Tuberculosis.Front Immunol. 2021 Feb 18;11:581911. doi: 10.3389/fimmu.2020.581911. eCollection 2020. Front Immunol. 2021. PMID: 33679685 Free PMC article.
-
Comparison of Biochemical and Hematological Profiles in Patients of Extrapulmonary and Pulmonary Tuberculosis at a Tertiary Care Center.Cureus. 2023 Mar 5;15(3):e35778. doi: 10.7759/cureus.35778. eCollection 2023 Mar. Cureus. 2023. PMID: 37025745 Free PMC article.
-
ACE2 in the second act of COVID-19 syndrome: Peptide dysregulation and possible correction with oestrogen.J Neuroendocrinol. 2021 Feb;33(2):e12935. doi: 10.1111/jne.12935. Epub 2021 Jan 18. J Neuroendocrinol. 2021. PMID: 33462852 Free PMC article. Review.
References
-
- WHO Global Tuberculosis Report. WHO; (2018).
LinkOut - more resources
Full Text Sources

