Pre-clinical evaluation of a quadrivalent HCV VLP vaccine in pigs following microneedle delivery
- PMID: 31239471
- PMCID: PMC6592879
- DOI: 10.1038/s41598-019-45461-z
Pre-clinical evaluation of a quadrivalent HCV VLP vaccine in pigs following microneedle delivery
Abstract
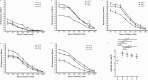
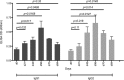
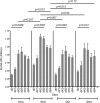
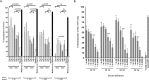
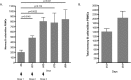
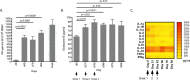
The introduction of directly acting antiviral agents (DAAs) has produced significant improvements in the ability to cure chronic hepatitis C infection. However, with over 2% of the world's population infected with HCV, complications arising from the development of cirrhosis of the liver, chronic hepatitis C infection remains the leading indication for liver transplantation. Several modelling studies have indicated that DAAs alone will not be sufficient to eliminate HCV, but if combined with an effective vaccine this regimen would provide a significant advance towards achieving this critical World Health Organisation goal. We have previously generated a genotype 1a, 1b, 2a, 3a HCV virus like particle (VLP) quadrivalent vaccine. The HCV VLPs contain the core and envelope proteins (E1 and E2) of HCV and the vaccine has been shown to produce broad humoral and T cell immune responses following vaccination of mice. In this report we further advanced this work by investigating vaccine responses in a large animal model. We demonstrate that intradermal microneedle vaccination of pigs with our quadrivalent HCV VLP based vaccine produces long-lived multi-genotype specific and neutralizing antibody (NAb) responses together with strong T cell and granzyme B responses and normal Th1 and Th2 cytokine responses. These responses were achieved without the addition of adjuvant. Our study demonstrates that our vaccine is able to produce broad immune responses in a large animal that, next to primates, is the closest animal model to humans. Our results are important as they show that the vaccine can produce robust immune responses in a large animal model before progressing the vaccine to human trials.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Immunological responses following administration of a genotype 1a/1b/2/3a quadrivalent HCV VLP vaccine.Sci Rep. 2018 Apr 24;8(1):6483. doi: 10.1038/s41598-018-24762-9. Sci Rep. 2018. PMID: 29691437 Free PMC article.
-
Antibody Responses to a Quadrivalent Hepatitis C Viral-Like Particle Vaccine Adjuvanted with Toll-Like Receptor 2 Agonists.Viral Immunol. 2018 May;31(4):338-343. doi: 10.1089/vim.2017.0182. Epub 2018 Feb 28. Viral Immunol. 2018. PMID: 29489437
-
A Recombinant Hepatitis C Virus Genotype 1a E1/E2 Envelope Glycoprotein Vaccine Elicits Antibodies That Differentially Neutralize Closely Related 2a Strains through Interactions of the N-Terminal Hypervariable Region 1 of E2 with Scavenger Receptor B1.J Virol. 2019 Oct 29;93(22):e00810-19. doi: 10.1128/JVI.00810-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462563 Free PMC article.
-
Antibody Responses in Hepatitis C Infection.Cold Spring Harb Perspect Med. 2021 Mar 1;11(3):a036962. doi: 10.1101/cshperspect.a036962. Cold Spring Harb Perspect Med. 2021. PMID: 32341067 Free PMC article. Review.
-
Hepatitis C Virus E1E2 Structure, Diversity, and Implications for Vaccine Development.Viruses. 2024 May 18;16(5):803. doi: 10.3390/v16050803. Viruses. 2024. PMID: 38793684 Free PMC article. Review.
Cited by
-
A panel of hepatitis C virus glycoproteins for the characterization of antibody responses using antibodies with diverse recognition and neutralization patterns.Virus Res. 2024 Mar;341:199308. doi: 10.1016/j.virusres.2024.199308. Epub 2024 Jan 11. Virus Res. 2024. PMID: 38171391 Free PMC article.
-
Skin-Based Vaccination: A Systematic Mapping Review of the Types of Vaccines and Methods Used and Immunity and Protection Elicited in Pigs.Vaccines (Basel). 2023 Feb 16;11(2):450. doi: 10.3390/vaccines11020450. Vaccines (Basel). 2023. PMID: 36851328 Free PMC article. Review.
-
Prospects for developing an Hepatitis C virus E1E2-based nanoparticle vaccine.Rev Med Virol. 2023 Sep;33(5):e2474. doi: 10.1002/rmv.2474. Epub 2023 Aug 11. Rev Med Virol. 2023. PMID: 37565536 Free PMC article. Review.
-
[Development approaches for vaccines against hepatitis C virus infections].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022 Feb;65(2):183-191. doi: 10.1007/s00103-021-03477-9. Epub 2022 Jan 11. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022. PMID: 35015104 Free PMC article. Review. German.
-
Current Hepatitis C Vaccine Candidates Based on the Induction of Neutralizing Antibodies.Viruses. 2023 May 11;15(5):1151. doi: 10.3390/v15051151. Viruses. 2023. PMID: 37243237 Free PMC article. Review.
References
-
- World Health Organization. Global Hepatitis Report 2017. Geneva: World Health Organization; 2017. Available at, http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?...., Accessed April, 2018. (2017).
-
- Division of Viral Hepatitis, N. C. f. H. A., Viral Hepatitis, STD, and TB Prevention. Progress toward viral hepatitis elimination in the United States, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

