Delayed Puberty-Phenotypic Diversity, Molecular Genetic Mechanisms, and Recent Discoveries
- PMID: 31220230
- PMCID: PMC6736054
- DOI: 10.1210/er.2018-00248
Delayed Puberty-Phenotypic Diversity, Molecular Genetic Mechanisms, and Recent Discoveries
Erratum in
-
ERRATUM FOR "Delayed Puberty-Phenotypic Diversity, Molecular Genetic Mechanisms, and Recent Discoveries".Endocr Rev. 2020 Feb 1;41(1):118. doi: 10.1210/endrev/bnz006. Endocr Rev. 2020. PMID: 31926007 Free PMC article. No abstract available.
Abstract
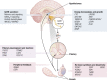
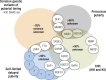
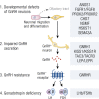
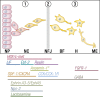
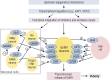
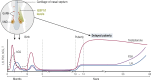
This review presents a comprehensive discussion of the clinical condition of delayed puberty, a common presentation to the pediatric endocrinologist, which may present both diagnostic and prognostic challenges. Our understanding of the genetic control of pubertal timing has advanced thanks to active investigation in this field over the last two decades, but it remains in large part a fascinating and mysterious conundrum. The phenotype of delayed puberty is associated with adult health risks and common etiologies, and there is evidence for polygenic control of pubertal timing in the general population, sex-specificity, and epigenetic modulation. Moreover, much has been learned from comprehension of monogenic and digenic etiologies of pubertal delay and associated disorders and, in recent years, knowledge of oligogenic inheritance in conditions of GnRH deficiency. Recently there have been several novel discoveries in the field of self-limited delayed puberty, encompassing exciting developments linking this condition to both GnRH neuronal biology and metabolism and body mass. These data together highlight the fascinating heterogeneity of disorders underlying this phenotype and point to areas of future research where impactful developments can be made.
Copyright © 2019 Endocrine Society.
Figures

Similar articles
-
Genes underlying delayed puberty.Mol Cell Endocrinol. 2018 Nov 15;476:119-128. doi: 10.1016/j.mce.2018.05.001. Epub 2018 May 4. Mol Cell Endocrinol. 2018. PMID: 29730183 Free PMC article. Review.
-
Genetic regulation in pubertal delay.J Mol Endocrinol. 2019 Oct;63(3):R37-R49. doi: 10.1530/JME-19-0130. J Mol Endocrinol. 2019. PMID: 31394496 Review.
-
The Genetic Basis of Delayed Puberty.Neuroendocrinology. 2018;106(3):283-291. doi: 10.1159/000481569. Epub 2017 Sep 18. Neuroendocrinology. 2018. PMID: 28926843 Review.
-
Genetics of pubertal timing.Best Pract Res Clin Endocrinol Metab. 2022 Jan;36(1):101618. doi: 10.1016/j.beem.2022.101618. Epub 2022 Feb 5. Best Pract Res Clin Endocrinol Metab. 2022. PMID: 35183440 Free PMC article. Review.
-
Genetics of pubertal timing.Curr Opin Pediatr. 2018 Aug;30(4):532-540. doi: 10.1097/MOP.0000000000000642. Curr Opin Pediatr. 2018. PMID: 29771761 Free PMC article. Review.
Cited by
-
Frasier Syndrome: A 15-Year-Old Phenotypically Female Adolescent Presenting with Delayed Puberty and Nephropathy.Children (Basel). 2023 Mar 17;10(3):577. doi: 10.3390/children10030577. Children (Basel). 2023. PMID: 36980135 Free PMC article.
-
Approach to the Patient: Management of Pituitary Hormone Replacement Through Transition.J Clin Endocrinol Metab. 2022 Jun 16;107(7):2077-2091. doi: 10.1210/clinem/dgac129. J Clin Endocrinol Metab. 2022. PMID: 35262704 Free PMC article.
-
POU6F2 mutation in humans with pubertal failure alters GnRH transcript expression.Front Endocrinol (Lausanne). 2023 Aug 1;14:1203542. doi: 10.3389/fendo.2023.1203542. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37600690 Free PMC article.
-
Interpretation of reproductive hormones before, during and after the pubertal transition-Identifying health and disordered puberty.Clin Endocrinol (Oxf). 2021 Nov;95(5):702-715. doi: 10.1111/cen.14578. Epub 2021 Aug 8. Clin Endocrinol (Oxf). 2021. PMID: 34368982 Free PMC article. Review.
-
Molecular and Environmental Mechanisms Regulating Puberty Initiation: An Integrated Approach.Front Endocrinol (Lausanne). 2019 Dec 6;10:828. doi: 10.3389/fendo.2019.00828. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31920956 Free PMC article. Review.
References
-
- Palmert MR, Boepple PA. Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab. 2001;86(6):2364–2368. - PubMed
-
- Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab. 1980;51(3):548–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

