A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus)
- PMID: 31205756
- PMCID: PMC6560728
- DOI: 10.1186/s41073-019-0069-3
A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus)
Abstract
Background: The ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines are widely endorsed but compliance is limited. We sought to determine whether journal-requested completion of an ARRIVE checklist improves full compliance with the guidelines.
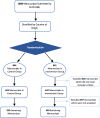
Methods: In a randomised controlled trial, manuscripts reporting in vivo animal research submitted to PLOS ONE (March-June 2015) were randomly allocated to either requested completion of an ARRIVE checklist or current standard practice. Authors, academic editors, and peer reviewers were blinded to group allocation. Trained reviewers performed outcome adjudication in duplicate by assessing manuscripts against an operationalised version of the ARRIVE guidelines that consists 108 items. Our primary outcome was the between-group differences in the proportion of manuscripts meeting all ARRIVE guideline checklist subitems.
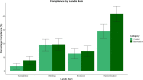
Results: We randomised 1689 manuscripts (control: n = 844, intervention: n = 845), of which 1269 were sent for peer review and 762 (control: n = 340; intervention: n = 332) accepted for publication. No manuscript in either group achieved full compliance with the ARRIVE checklist. Details of animal husbandry (ARRIVE subitem 9b) was the only subitem to show improvements in reporting, with the proportion of compliant manuscripts rising from 52.1 to 74.1% (X 2 = 34.0, df = 1, p = 2.1 × 10-7) in the control and intervention groups, respectively.
Conclusions: These results suggest that altering the editorial process to include requests for a completed ARRIVE checklist is not enough to improve compliance with the ARRIVE guidelines. Other approaches, such as more stringent editorial policies or a targeted approach on key quality items, may promote improvements in reporting.
Keywords: ARRIVE; Randomised controlled trial; Reporting guidelines.
Conflict of interest statement
Competing interestsES and MM are in receipt of competitive research grants from the NC3Rs who developed the ARRIVE guidelines. SL was funded by an NC3Rs PhD studentship. ES, MM, DH, and NK are members of an NC3Rs working group to review the ARRIVE guidelines (https://www.nc3rs.org.uk/revision-arrive-guidelines). CM, GM, AC, GA, and MD were all editors at PLOS ONE throughout the duration of the study. ES is Editor-in-Chief at BMJ Open Science. All other authors have no other competing interests to declare.
Figures

Similar articles
-
Effect of an editorial intervention to improve the completeness of reporting of randomised trials: a randomised controlled trial.BMJ Open. 2020 May 18;10(5):e036799. doi: 10.1136/bmjopen-2020-036799. BMJ Open. 2020. PMID: 32430454 Free PMC article. Clinical Trial.
-
The ARRIVE guidelines 2.0: updated guidelines for reporting animal research.J Physiol. 2020 Sep;598(18):3793-3801. doi: 10.1113/JP280389. Epub 2020 Jul 14. J Physiol. 2020. PMID: 32666574 Free PMC article.
-
The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research.PLoS Biol. 2020 Jul 14;18(7):e3000410. doi: 10.1371/journal.pbio.3000410. eCollection 2020 Jul. PLoS Biol. 2020. PMID: 32663219 Free PMC article.
-
Reporting in rodent models of 'chemobrain': a systematic review assessing compliance with the ARRIVE guidelines.Support Care Cancer. 2021 Nov;29(11):7073-7084. doi: 10.1007/s00520-021-06312-8. Epub 2021 Jun 2. Support Care Cancer. 2021. PMID: 34080055 Review.
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article. Review.
Cited by
-
The ARRIVE 2.0 Guidelines: Importance and Full Adoption by AALAS Journals.J Am Assoc Lab Anim Sci. 2024 Sep 5;63(5):449-54. doi: 10.30802/AALAS-JAALAS-24-083. Online ahead of print. J Am Assoc Lab Anim Sci. 2024. PMID: 39237288 No abstract available.
-
A guide to open science practices for animal research.PLoS Biol. 2022 Sep 15;20(9):e3001810. doi: 10.1371/journal.pbio.3001810. eCollection 2022 Sep. PLoS Biol. 2022. PMID: 36108043 Free PMC article.
-
The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research.BMC Vet Res. 2020 Jul 14;16(1):242. doi: 10.1186/s12917-020-02451-y. BMC Vet Res. 2020. PMID: 32660541 Free PMC article.
-
Recommendations for robust and reproducible preclinical research in personalised medicine.BMC Med. 2023 Jan 8;21(1):14. doi: 10.1186/s12916-022-02719-0. BMC Med. 2023. PMID: 36617553 Free PMC article. Review.
-
Risk of bias assessment in preclinical literature using natural language processing.Res Synth Methods. 2022 May;13(3):368-380. doi: 10.1002/jrsm.1533. Epub 2021 Nov 5. Res Synth Methods. 2022. PMID: 34709718 Free PMC article.
References
-
- Avey MT, Moher D, Sullivan KJ, Fergusson D, Griffin G, Grimshaw JM, Hutton B, Lalu MM, Macleod M, Marshall J, Mei SHJ, Rudnicki M, Stewart DJ, Turgeon AF, Mcintyre L, Canadian Critical Care Translational Biology, G The devil is in the details: incomplete reporting in preclinical animal research. PLoS One. 2016;11:e0166733. doi: 10.1371/journal.pone.0166733. - DOI - PMC - PubMed
-
- Cobo E, Cortes J, Ribera JM, Cardellach F, Selva-O’Callaghan A, Kostov B, Garcia L, Cirugeda L, Altman DG, Gonzalez JA, Sanchez JA, Miras F, Urrutia A, Fonollosa V, Rey-Joly C, Vilardell M. Effect of using reporting guidelines during peer review on quality of final manuscripts submitted to a biomedical journal: masked randomised trial. BMJ. 2011;343:d6783. doi: 10.1136/bmj.d6783. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

