Olaparib as maintenance treatment for patients with platinum-sensitive relapsed ovarian cancer
- PMID: 31205507
- PMCID: PMC6535754
- DOI: 10.1177/1758835919849753
Olaparib as maintenance treatment for patients with platinum-sensitive relapsed ovarian cancer
Abstract
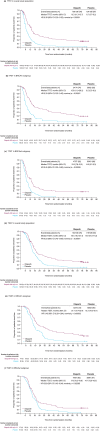
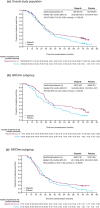
Poly (ADP-ribose) polymerase (PARP) inhibitors were developed with the intention of treating patients with homologous recombination repair deficiency (HRD), specifically for patients with tumours that harbour a BRCA mutation (BRCAm). Evidence from clinical trials to date has demonstrated that patients with a BRCAm derive the greatest benefit from PARP inhibitors. However, clinical studies have also shown that PARP inhibitors provide benefit to women with ovarian cancer who do not have a BRCAm. The recent updated approvals of olaparib, niraparib and rucaparib by the US Food and Drug Administration and the European Medicines Agency for the treatment of all platinum-sensitive relapsed (PSR) ovarian-cancer populations, regardless of their BRCAm status, support this. Long-term tolerability and efficacy of olaparib have been demonstrated in patients both with and without a BRCAm, with 13% of patients receiving maintenance olaparib for at least 5 years in one study, which is unprecedented in the relapsed ovarian-cancer setting (versus 1% on placebo). Further studies should be performed to elucidate which non-BRCAm patients are deriving benefit and what molecular processes are enabling this, so that patients continue to receive optimal treatment for their disease. Here, we review clinical and molecular markers of HRD, the long-term clinical safety and efficacy of PARP inhibitors in ovarian cancer, with a focus on olaparib and the current approved indications for PARP inhibitors, as well as guidance on treatment decisions for patients with PSR ovarian cancer.
Keywords: PARP inhibitor; niraparib; olaparib; platinum-sensitive relapsed ovarian cancer; rucaparib; targeted therapy.
Conflict of interest statement
Conflict of interest statement: Jonathan A. Ledermann has attended AstraZeneca, Clovis Oncology, Pfizer and Roche Advisory Board Meetings and received lecture fees from these companies. He has received research funding from AstraZeneca and Merck/MSD. Eric Pujade-Lauraine has attended AstraZeneca, Clovis, Pfizer, Roche and Tesaro Advisory Board Meetings, has received travel support from AstraZeneca, Roche and Tesaro, is Chair for ARCAGY Research and is a consultant for Menarini.
Figures

Similar articles
-
Molecular and clinical predictors of improvement in progression-free survival with maintenance PARP inhibitor therapy in women with platinum-sensitive, recurrent ovarian cancer: A meta-analysis.Cancer. 2021 Jul 15;127(14):2432-2441. doi: 10.1002/cncr.33517. Epub 2021 Mar 19. Cancer. 2021. PMID: 33740262
-
Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial.Lancet Oncol. 2016 Nov;17(11):1579-1589. doi: 10.1016/S1470-2045(16)30376-X. Epub 2016 Sep 9. Lancet Oncol. 2016. PMID: 27617661 Clinical Trial.
-
Olaparib maintenance therapy in patients with platinum-sensitive, relapsed serous ovarian cancer and a BRCA mutation: Overall survival adjusted for postprogression poly(adenosine diphosphate ribose) polymerase inhibitor therapy.Cancer. 2016 Jun 15;122(12):1844-52. doi: 10.1002/cncr.29995. Epub 2016 Apr 8. Cancer. 2016. PMID: 27062051 Clinical Trial.
-
What Is the Place of PARP Inhibitors in Ovarian Cancer Treatment?Curr Oncol Rep. 2016 May;18(5):29. doi: 10.1007/s11912-016-0515-z. Curr Oncol Rep. 2016. PMID: 26984416 Review.
-
Using PARP Inhibitors in the Treatment of Patients With Ovarian Cancer.Curr Treat Options Oncol. 2018 Nov 15;19(12):1. doi: 10.1007/s11864-018-0572-7. Curr Treat Options Oncol. 2018. PMID: 30535808 Free PMC article. Review.
Cited by
-
Glutathione Transferase P1: Potential Therapeutic Target in Ovarian Cancer.Medicina (Kaunas). 2022 Nov 16;58(11):1660. doi: 10.3390/medicina58111660. Medicina (Kaunas). 2022. PMID: 36422199 Free PMC article. Review.
-
Current and Emerging Molecular Therapies for Head and Neck Squamous Cell Carcinoma.Cancers (Basel). 2021 Oct 30;13(21):5471. doi: 10.3390/cancers13215471. Cancers (Basel). 2021. PMID: 34771633 Free PMC article. Review.
-
PARP Inhibitors as Therapeutics: Beyond Modulation of PARylation.Cancers (Basel). 2020 Feb 8;12(2):394. doi: 10.3390/cancers12020394. Cancers (Basel). 2020. PMID: 32046300 Free PMC article. Review.
-
PARP inhibitor synthetic lethality in ATM biallelic mutant cancer cell lines is associated with BRCA1/2 and RAD51 downregulation.Front Oncol. 2024 May 14;14:1380633. doi: 10.3389/fonc.2024.1380633. eCollection 2024. Front Oncol. 2024. PMID: 38807759 Free PMC article.
-
The function and regulation of ADP-ribosylation in the DNA damage response.Biochem Soc Trans. 2023 Jun 28;51(3):995-1008. doi: 10.1042/BST20220749. Biochem Soc Trans. 2023. PMID: 37171085 Free PMC article.
References
-
- European Medicines Agency. Lynparza recommended for approval in ovarian cancer. Press release, http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2... (2014, accessed 3 July 2018).
-
- Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med 2016; 8: 362ps17. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

