Tumor Size and Overall Survival in Patients With Platinum-Resistant Ovarian Cancer Treated With Chemotherapy and Bevacizumab
- PMID: 31191068
- PMCID: PMC6540487
- DOI: 10.1177/1179554919852071
Tumor Size and Overall Survival in Patients With Platinum-Resistant Ovarian Cancer Treated With Chemotherapy and Bevacizumab
Abstract
Introduction: Ovarian cancer is now recognized as a constellation of distinct subtypes of neoplasia involving the ovary and related structures. As a consequence of this heterogeneity, the analysis of covariates influencing the overall survival is crucial in this disease segment. In this work, an overall survival model incorporating tumor kinetics metrics in patients with platinum-resistant ovarian cancer was developed from the randomized, open label, phase 3 AURELIA trial.
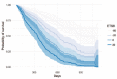
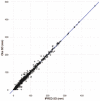
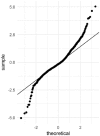
Methods: Tumor size data from 361 patients randomly allocated to the bevacizumab + chemotherapy or chemotherapy study arm were collected at baseline and every 8 to 9 weeks until disease progression. Patients continued to be followed for survival after treatment discontinuation. A landmarked Cox proportional hazard survival model was developed to characterize the overall survival distribution.
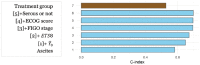
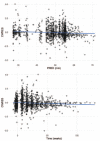
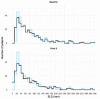
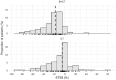
Results: Two sets of factors were found to be influential on survival time: those describing the type and severity of disease (Eastern Cooperative Oncology Group [ECOG], Féderation Internationale de Gynécologie et d'Obstétrique [FIGO] stages, presence of ascites) and those summarizing the key features of the tumor kinetic model (tumor shrinkage at week 8 and tumor size at treatment onset). The treatment group was not required in the final model as the drug effect was accounted for in the tumor kinetics model.
Conclusions: This work has identified both ascites and tumor kinetics metrics as being the 2 most influential factors to explain variability in overall survival in patients with platinum-resistant ovarian cancer.
Keywords: angiogenesis; anti-angiogenesis; ovarian cancer; overall survival; therapy; tumor kinetics.
Conflict of interest statement
Declaration Of Conflicting Interests:The author(s) declared following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Alexandre Sostelly and François Mercier were employed by F. Hofmann-La Roche Ltd.
Figures

Similar articles
-
Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial.Lancet Oncol. 2015 Aug;16(8):928-36. doi: 10.1016/S1470-2045(15)00086-8. Epub 2015 Jun 23. Lancet Oncol. 2015. PMID: 26115797 Free PMC article. Clinical Trial.
-
Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2021 Apr;22(4):439-449. doi: 10.1016/S1470-2045(21)00006-1. Epub 2021 Mar 8. Lancet Oncol. 2021. PMID: 33705695 Clinical Trial.
-
An open-label, randomized, phase II trial evaluating the efficacy and safety of standard of care with or without bevacizumab in platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer patients previously treated with bevacizumab for front-line or platinum-sensitive ovarian cancer: rationale, design, and methods of the Japanese Gynecologic Oncology Group study JGOG3023.BMC Cancer. 2018 Jul 31;18(1):771. doi: 10.1186/s12885-018-4505-4. BMC Cancer. 2018. PMID: 30064406 Free PMC article. Clinical Trial.
-
Clinical benefit of controversial first line systemic therapies for advanced stage ovarian cancer - ESMO-MCBS scores.Cancer Treat Rev. 2018 Sep;69:233-242. doi: 10.1016/j.ctrv.2018.06.008. Epub 2018 Jun 18. Cancer Treat Rev. 2018. PMID: 30098485 Review.
-
A New Therapeutic Strategy for Recurrent Ovarian Cancer-Bevacizumab beyond Progressive Disease.Healthcare (Basel). 2019 Sep 19;7(3):109. doi: 10.3390/healthcare7030109. Healthcare (Basel). 2019. PMID: 31546963 Free PMC article. Review.
Cited by
-
Surgery in platinum-resistant recurrent epithelial ovarian carcinoma.World J Clin Cases. 2022 Apr 26;10(12):3739-3753. doi: 10.12998/wjcc.v10.i12.3739. World J Clin Cases. 2022. PMID: 35647161 Free PMC article.
-
Early predictions of response and survival from a tumor dynamics model in patients with recurrent, metastatic head and neck squamous cell carcinoma treated with immunotherapy.CPT Pharmacometrics Syst Pharmacol. 2021 Mar;10(3):230-240. doi: 10.1002/psp4.12594. Epub 2021 Feb 13. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 33465293 Free PMC article. Clinical Trial.
-
Bevacizumab Combined with Chemotherapy in Platinum-Resistant Ovarian Cancer: Beyond the AURELIA Trial.Transl Cancer Res. 2020 Apr;9(4):2164-2167. doi: 10.21037/tcr.2020.02.42. Transl Cancer Res. 2020. PMID: 34113549 Free PMC article. No abstract available.
-
Expression of PAWR predicts prognosis of ovarian cancer.Cancer Cell Int. 2020 Dec 14;20(1):598. doi: 10.1186/s12935-020-01704-y. Cancer Cell Int. 2020. PMID: 33317551 Free PMC article.
-
Current Ovarian Cancer Maintenance Strategies and Promising New Developments.J Cancer. 2021 Jan 1;12(1):38-53. doi: 10.7150/jca.49406. eCollection 2021. J Cancer. 2021. PMID: 33391401 Free PMC article. Review.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. - PubMed
-
- Sridhara R, Mandrekar SJ, Dodd LE. Missing data and measurement variability in assessing progression-free survival endpoint in randomized clinical trials. Clin Cancer Res. 2013;19:2613–2620. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

