A Phase I Trial of the VEGF Receptor Tyrosine Kinase Inhibitor Pazopanib in Combination with the MEK Inhibitor Trametinib in Advanced Solid Tumors and Differentiated Thyroid Cancers
- PMID: 31186313
- PMCID: PMC7038784
- DOI: 10.1158/1078-0432.CCR-18-1881
A Phase I Trial of the VEGF Receptor Tyrosine Kinase Inhibitor Pazopanib in Combination with the MEK Inhibitor Trametinib in Advanced Solid Tumors and Differentiated Thyroid Cancers
Abstract
Purpose: Differentiated thyroid cancer (DTC) responds to VEGF receptor inhibitors. VEGF signals through RAS/RAF/MEK signaling. We evaluated the safety and efficacy of the VEGF receptor inhibitor pazopanib and MEK inhibitor trametinib in advanced solid tumors and DTC.
Patients and methods: Patients with advanced solid tumors were enrolled in a phase I, multicenter trial with a DTC expansion cohort. Patients received pazopanib 400-800 mg and trametinib 1-2 mg daily. Efficacy in the expansion cohort was assessed with objective response (OR) at 6 months of treatment.
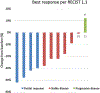
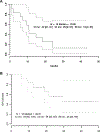
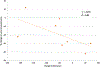
Results: Twenty-six patients were enrolled in five dose levels. MTD was not reached; the recommended phase II dose was pazopanib 800 mg orally and trametinib 2 mg orally every day. There was one dose-limiting toxicity on dose level 1 with grade 3 fatigue and muscle weakness. Common grade 3 adverse events were elevated transaminases (19%), diarrhea (15%), hypertension (12%), and fatigue (8%). Thirteen patients were enrolled in the DTC cohort; OR was 33% (95% confidence interval, 9.9, 65.1%) and median progression-free survival was 10.7 months. The cohort was terminated after planned interim analysis suggested insufficiently increased activity against the historical control of pazopanib alone. Reduction in tumor diameter negatively correlated with p-ERK change in tumor (Spearman ρ = -0.71; P = 0.05). NRAS mutation was associated with response (Fisher exact P = 0.008).
Conclusions: Pazopanib + trametinib was tolerable at full single-agent doses with clinical activity in DTC but did not achieve the prespecified response rate target.
©2019 American Association for Cancer Research.
Figures
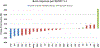
Similar articles
-
The oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma.Br J Cancer. 2017 May 23;116(11):1402-1407. doi: 10.1038/bjc.2017.119. Epub 2017 Apr 25. Br J Cancer. 2017. PMID: 28441383 Free PMC article.
-
Phase Ib/II Study of the Safety and Efficacy of Combination Therapy with Multikinase VEGF Inhibitor Pazopanib and MEK Inhibitor Trametinib In Advanced Soft Tissue Sarcoma.Clin Cancer Res. 2017 Aug 1;23(15):4027-4034. doi: 10.1158/1078-0432.CCR-17-0272. Epub 2017 Apr 4. Clin Cancer Res. 2017. PMID: 28377484 Free PMC article. Clinical Trial.
-
Trametinib with and without pazopanib has potent preclinical activity in thyroid cancer.Oncol Rep. 2015 Nov;34(5):2319-24. doi: 10.3892/or.2015.4225. Epub 2015 Aug 26. Oncol Rep. 2015. PMID: 26324075 Free PMC article.
-
Lenvatinib: Role in thyroid cancer and other solid tumors.Cancer Treat Rev. 2016 Jan;42:47-55. doi: 10.1016/j.ctrv.2015.11.003. Epub 2015 Dec 2. Cancer Treat Rev. 2016. PMID: 26678514 Review.
-
Evolving approaches to patients with advanced differentiated thyroid cancer.Endocr Rev. 2013 Jun;34(3):439-55. doi: 10.1210/er.2012-1038. Epub 2013 Apr 10. Endocr Rev. 2013. PMID: 23575762 Free PMC article. Review.
Cited by
-
Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy.J Hematol Oncol. 2022 Jul 7;15(1):89. doi: 10.1186/s13045-022-01310-7. J Hematol Oncol. 2022. PMID: 35799213 Free PMC article. Review.
-
New Therapies for Advanced Thyroid Cancer.Front Endocrinol (Lausanne). 2020 May 22;11:82. doi: 10.3389/fendo.2020.00082. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32528402 Free PMC article. Review.
-
Current Perspectives and Novel Strategies of NRAS-Mutant Melanoma.Onco Targets Ther. 2021 Jun 9;14:3709-3719. doi: 10.2147/OTT.S278095. eCollection 2021. Onco Targets Ther. 2021. PMID: 34135599 Free PMC article. Review.
-
Novel Therapeutics in Radioactive Iodine-Resistant Thyroid Cancer.Front Endocrinol (Lausanne). 2021 Jul 15;12:720723. doi: 10.3389/fendo.2021.720723. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34335481 Free PMC article. Review.
-
Prognostic and Predictive Molecular Markers in Cholangiocarcinoma.Cancers (Basel). 2022 Feb 17;14(4):1026. doi: 10.3390/cancers14041026. Cancers (Basel). 2022. PMID: 35205774 Free PMC article. Review.
References
-
- Abdel-Rahman O Targeting vascular endothelial growth factor (VEGF) pathway in iodine-refractory differentiated thyroid carcinoma (DTC): from bench to bedside. Crit Rev Oncol Hematol 2015;94:45–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

