Differential cleavage of lysyl oxidase by the metalloproteinases BMP1 and ADAMTS2/14 regulates collagen binding through a tyrosine sulfate domain
- PMID: 31152061
- PMCID: PMC6643040
- DOI: 10.1074/jbc.RA119.007806
Differential cleavage of lysyl oxidase by the metalloproteinases BMP1 and ADAMTS2/14 regulates collagen binding through a tyrosine sulfate domain
Abstract
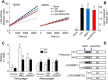
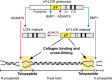
Collagens are the main structural component of the extracellular matrix and provide biomechanical properties to connective tissues. A critical step in collagen fibril formation is the proteolytic removal of N- and C-terminal propeptides from procollagens by metalloproteinases of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) and BMP1 (bone morphogenetic protein 1)/Tolloid-like families, respectively. BMP1 also cleaves and activates the lysyl oxidase (LOX) precursor, the enzyme catalyzing the initial step in the formation of covalent collagen cross-links, an essential process for fibril stabilization. In this study, using murine skin fibroblasts and HEK293 cells, along with immunoprecipitation, LOX enzymatic activity, solid-phase binding assays, and proteomics analyses, we report that the LOX precursor is proteolytically processed by the procollagen N-proteinases ADAMTS2 and ADAMTS14 between Asp-218 and Tyr-219, 50 amino acids downstream of the BMP1 cleavage site. We noted that the LOX sequence between the BMP1- and ADAMTS-processing sites contains several conserved tyrosine residues, of which some are post-translationally modified by tyrosine O-sulfation and contribute to binding to collagen. Taken together, these findings unravel an additional level of regulation in the formation of collagen fibrils. They point to a mechanism that controls the binding of LOX to collagen and is based on differential BMP1- and ADAMTS2/14-mediated cleavage of a tyrosine-sulfated domain.
Keywords: ADAMTS; collagen; connective tissue; extracellular matrix; lysyl oxidase; metalloproteinase; post-translational modification (PTM); proteolysis; tyrosine sulfation.
© 2019 Rosell-García et al.
Conflict of interest statement
F. R.-P. reports grants from Pharmaxis, outside the submitted work
Figures



Similar articles
-
Cleavage of LOXL1 by BMP1 and ADAMTS14 Proteases Suggests a Role for Proteolytic Processing in the Regulation of LOXL1 Function.Int J Mol Sci. 2022 Mar 18;23(6):3285. doi: 10.3390/ijms23063285. Int J Mol Sci. 2022. PMID: 35328709 Free PMC article.
-
Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures.J Biol Chem. 2001 Jun 22;276(25):22537-43. doi: 10.1074/jbc.M102352200. Epub 2001 Apr 19. J Biol Chem. 2001. PMID: 11313359
-
Enhancement of collagen deposition and cross-linking by coupling lysyl oxidase with bone morphogenetic protein-1 and its application in tissue engineering.Sci Rep. 2018 Jul 17;8(1):10780. doi: 10.1038/s41598-018-29236-6. Sci Rep. 2018. PMID: 30018337 Free PMC article.
-
Developmental roles of the BMP1/TLD metalloproteinases.Birth Defects Res C Embryo Today. 2006 Mar;78(1):47-68. doi: 10.1002/bdrc.20060. Birth Defects Res C Embryo Today. 2006. PMID: 16622848 Review.
-
Lysyl oxidases: from enzyme activity to extracellular matrix cross-links.Essays Biochem. 2019 Sep 13;63(3):349-364. doi: 10.1042/EBC20180050. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31488698 Review.
Cited by
-
The Regulation of Collagen Processing by miRNAs in Disease and Possible Implications for Bone Turnover.Int J Mol Sci. 2021 Dec 22;23(1):91. doi: 10.3390/ijms23010091. Int J Mol Sci. 2021. PMID: 35008515 Free PMC article. Review.
-
Cleavage of LOXL1 by BMP1 and ADAMTS14 Proteases Suggests a Role for Proteolytic Processing in the Regulation of LOXL1 Function.Int J Mol Sci. 2022 Mar 18;23(6):3285. doi: 10.3390/ijms23063285. Int J Mol Sci. 2022. PMID: 35328709 Free PMC article.
-
Mechanistic insight into lysyl oxidase in vascular remodeling and angiogenesis.Genes Dis. 2022 May 26;10(3):771-785. doi: 10.1016/j.gendis.2022.05.011. eCollection 2023 May. Genes Dis. 2022. PMID: 37396555 Free PMC article. Review.
-
Knock-Out of the Five Lysyl-Oxidase Family Genes Enables Identification of Lysyl-Oxidase Pro-Enzyme Regulated Genes.Int J Mol Sci. 2022 Sep 26;23(19):11322. doi: 10.3390/ijms231911322. Int J Mol Sci. 2022. PMID: 36232621 Free PMC article.
-
Functions and Mechanisms of Pro-Lysyl Oxidase Processing in Cancers and Eye Pathologies with a Focus on Diabetic Retinopathy.Int J Mol Sci. 2022 May 3;23(9):5088. doi: 10.3390/ijms23095088. Int J Mol Sci. 2022. PMID: 35563478 Free PMC article. Review.
References
-
- Engel J., and Chiquet M. (2011) in The Extracellular Matrix: an Overview (Mecham R. P., ed) pp. 1–39, Springer-Verlag, Berlin, Heidelberg
-
- Eyre D. R., and Wu J. J. (2005) Collagen cross-links. Top. Curr. Chem. 247, 207–229 10.1007/b103828 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

