MicroRNAs in Microglia: How do MicroRNAs Affect Activation, Inflammation, Polarization of Microglia and Mediate the Interaction Between Microglia and Glioma?
- PMID: 31133802
- PMCID: PMC6522842
- DOI: 10.3389/fnmol.2019.00125
MicroRNAs in Microglia: How do MicroRNAs Affect Activation, Inflammation, Polarization of Microglia and Mediate the Interaction Between Microglia and Glioma?
Abstract
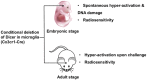
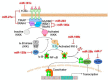
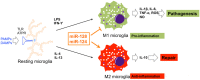
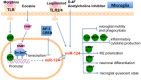
The essential roles of microglia in maintaining homeostasis in the healthy brain and contributing to neuropathology are well documented. Emerging evidence suggests that epigenetic modulation regulates microglial behavior in both physiological and pathological conditions. MicroRNAs (miRNAs) are short, non-coding epigenetic regulators that repress target gene expression mostly via binding to 3'-untranslated region (3'-UTR) of mRNA in a Dicer-dependent manner. Dysregulation of certain miRNAs can contribute to microglial hyper-activation, persistent neuroinflammation, and abnormal macrophage polarization in the brain. These abnormal conditions can support the pathogenesis of neurological disorders such as glioma, Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), stroke, ischemia, and spinal cord injury (SCI). However, the roles of miRNAs in microglia in health and neurological disease have not been systematically summarized. This review will first report the role of Dicer, a key endoribonulease that is responsible for most miRNA biogenesis in microglia. Second, we will focus on recent research about the function of miRNAs in activation, inflammation and polarization of microglia, respectively. In addition, potential crosstalk between microglia and glioma cells via miRNAs will be discussed in this part. Finally, the role of two essential miRNAs, miR-124, and miR-155, in microglia will be highlighted.
Keywords: activation; glioma; inflammation; microRNA; microglia; polarization.
Figures

Similar articles
-
MicroRNAs: Key Players in Microglia and Astrocyte Mediated Inflammation in CNS Pathologies.Curr Med Chem. 2016;23(30):3528-3546. doi: 10.2174/0929867323666160814001040. Curr Med Chem. 2016. PMID: 27528056 Review.
-
MicroRNAs in microglia: deciphering their role in neurodegenerative diseases.Front Cell Neurosci. 2024 May 15;18:1391537. doi: 10.3389/fncel.2024.1391537. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38812793 Free PMC article. Review.
-
miRNAs in Microglia: Important Players in Multiple Sclerosis Pathology.ASN Neuro. 2021 Jan-Dec;13:1759091420981182. doi: 10.1177/1759091420981182. ASN Neuro. 2021. PMID: 33517686 Free PMC article. Review.
-
Potential of activated microglia as a source of dysregulated extracellular microRNAs contributing to neurodegeneration in amyotrophic lateral sclerosis.J Neuroinflammation. 2020 Apr 28;17(1):135. doi: 10.1186/s12974-020-01822-4. J Neuroinflammation. 2020. PMID: 32345319 Free PMC article. Review.
-
Emerging role of non-coding RNAs in neuroinflammation mediated by microglia and astrocytes.J Neuroinflammation. 2023 Jul 23;20(1):173. doi: 10.1186/s12974-023-02856-0. J Neuroinflammation. 2023. PMID: 37481642 Free PMC article. Review.
Cited by
-
MicroRNAs Instruct and Maintain Cell Type Diversity in the Nervous System.Front Mol Neurosci. 2021 Apr 29;14:646072. doi: 10.3389/fnmol.2021.646072. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33994943 Free PMC article. Review.
-
Transcriptomic Profiling of Primary Microglia: Effects of miR-19a-3p and miR-19b-3p on Microglia Activation.Int J Mol Sci. 2024 Oct 1;25(19):10601. doi: 10.3390/ijms251910601. Int J Mol Sci. 2024. PMID: 39408930 Free PMC article.
-
Microglial Activation in Metal Neurotoxicity: Impact in Neurodegenerative Diseases.Biomed Res Int. 2023 Jan 31;2023:7389508. doi: 10.1155/2023/7389508. eCollection 2023. Biomed Res Int. 2023. PMID: 36760476 Free PMC article. Review.
-
MiR-30a-5p and miR-153-3p regulate LPS-induced neuroinflammatory response and neuronal apoptosis by targeting NeuroD1.BMB Rep. 2022 Sep;55(9):447-452. doi: 10.5483/BMBRep.2022.55.9.061. BMB Rep. 2022. PMID: 35651331 Free PMC article.
-
Loss of microRNA-15a/16-1 function promotes neuropathological and functional recovery in experimental traumatic brain injury.JCI Insight. 2024 Jun 24;9(12):e178650. doi: 10.1172/jci.insight.178650. JCI Insight. 2024. PMID: 38912585 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

