A versatile high-throughput assay to characterize antibody-mediated neutrophil phagocytosis
- PMID: 31132351
- PMCID: PMC6620195
- DOI: 10.1016/j.jim.2019.05.006
A versatile high-throughput assay to characterize antibody-mediated neutrophil phagocytosis
Abstract
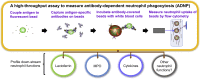
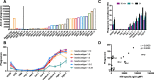
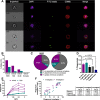
Neutrophils, the most abundant white blood cell, play a critical role in anti-pathogen immunity via phagocytic clearance, secretion of enzymes and immunomodulators, and the release of extracellular traps. Neutrophils non-specifically sense infection through an array of innate immune receptors and inflammatory sensors, but are also able to respond in a pathogen/antigen-specific manner when leveraged by antibodies via Fc-receptors. Among neutrophil functions, antibody-dependent neutrophil phagocytosis (ADNP) results in antibody-mediated opsonization, enabling neutrophils to sense and respond to infection in a pathogen-appropriate manner. Here, we describe a high-throughput flow cytometric approach to effectively visualize and quantify ADNP and its downstream consequences. The assay is easily adaptable, supporting both the use of purified neutrophils or white blood cells, the use of purified Ig or serum, and the broad utility of any target antigen. Thus, this ADNP assay represents a high-throughput platform for the in-depth characterization of neutrophil function.
Keywords: Antibody-dependent neutrophil phagocytosis; High-throughput assay; Immune correlates of protection; Non-neutralizing antibody; Systems serology.
Copyright © 2019. Published by Elsevier B.V.
Figures

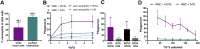



Similar articles
-
Determining the phagocytic activity of clinical antibody samples.J Vis Exp. 2011 Nov 30;(57):e3588. doi: 10.3791/3588. J Vis Exp. 2011. PMID: 22143444 Free PMC article.
-
Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner.mBio. 2016 Oct 4;7(5):e01624-16. doi: 10.1128/mBio.01624-16. mBio. 2016. PMID: 27703076 Free PMC article.
-
Neutrophils mediate HIV-specific antibody-dependent phagocytosis and ADCC.J Immunol Methods. 2018 Jun;457:41-52. doi: 10.1016/j.jim.2018.03.007. Epub 2018 Mar 29. J Immunol Methods. 2018. PMID: 29605231
-
A novel method for high-throughput detection and quantification of neutrophil extracellular traps reveals ROS-independent NET release with immune complexes.Autoimmun Rev. 2016 Jun;15(6):577-84. doi: 10.1016/j.autrev.2016.02.018. Epub 2016 Feb 27. Autoimmun Rev. 2016. PMID: 26925759 Review.
-
Human neutrophils in auto-immunity.Semin Immunol. 2016 Apr;28(2):159-73. doi: 10.1016/j.smim.2016.03.004. Epub 2016 Mar 29. Semin Immunol. 2016. PMID: 27036091 Review.
Cited by
-
COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women.Sci Transl Med. 2021 Oct 27;13(617):eabi8631. doi: 10.1126/scitranslmed.abi8631. Epub 2021 Oct 27. Sci Transl Med. 2021. PMID: 34664972 Free PMC article.
-
mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern.Immunity. 2022 Feb 8;55(2):355-365.e4. doi: 10.1016/j.immuni.2022.01.001. Epub 2022 Jan 6. Immunity. 2022. PMID: 35090580 Free PMC article.
-
Protective antibodies elicited by SARS-CoV-2 spike protein vaccination are boosted in the lung after challenge in nonhuman primates.Sci Transl Med. 2021 Aug 18;13(607):eabi4547. doi: 10.1126/scitranslmed.abi4547. Epub 2021 Jul 27. Sci Transl Med. 2021. PMID: 34315825 Free PMC article.
-
Compromised SARS-CoV-2-specific placental antibody transfer.Cell. 2021 Feb 4;184(3):628-642.e10. doi: 10.1016/j.cell.2020.12.027. Epub 2020 Dec 23. Cell. 2021. PMID: 33476549 Free PMC article.
-
Persistence of viral RNA in lymph nodes in ART-suppressed SIV/SHIV-infected Rhesus Macaques.Nat Commun. 2021 Mar 5;12(1):1474. doi: 10.1038/s41467-021-21724-0. Nat Commun. 2021. PMID: 33674572 Free PMC article.
References
-
- Ackerman M.E., Moldt B., Wyatt R.T., Dugast A.S., McAndrew E., Tsoukas S., Jost S., Berger C.T., Sciaranghella G., Liu Q., Irvine D.J., Burton D.R., Alter G. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J. Immunol. Methods. 2011;366:8–19. - PMC - PubMed
- Ackerman, M. E., B. Moldt, R. T. Wyatt, A. S. Dugast, E. McAndrew, S. Tsoukas, S. Jost, C. T. Berger, G. Sciaranghella, Q. Liu, D. J. Irvine, D. R. Burton, and G. Alter. 2011. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 366: 8-19. - PMC - PubMed
-
- Ackerman M.E., Mikhailova A., Brown E.P., Dowell K.G., Walker B.D., Bailey-Kellogg C., Suscovich T.J., Alter G. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog. 2016;12 - PMC - PubMed
- Ackerman, M. E., A. Mikhailova, E. P. Brown, K. G. Dowell, B. D. Walker, C. Bailey-Kellogg, T. J. Suscovich, and G. Alter. 2016. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog 12: e1005315. - PMC - PubMed
-
- Ackerman M.E., Das J., Pittala S., Broge T., Linde C., Suscovich T.J., Brown E.P., Bradley T., Natarajan H., Lin S., Sassic J.K., O'Keefe S., Mehta N., Goodman D., Sips M., Weiner J.A., Tomaras G.D., Haynes B.F., Lauffenburger D.A., Bailey-Kellogg C., Roederer M., Alter G. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat. Med. 2018 Oct;24(10):1590–1598. Epub 2018 Sep 3. - PMC - PubMed
- Ackerman, M. E., J. Das, S. Pittala, T. Broge, C. Linde, T. J. Suscovich, E. P. Brown, T. Bradley, H. Natarajan, S. Lin, J. K. Sassic, S. O'Keefe, N. Mehta, D. Goodman, M. Sips, J. A. Weiner, G. D. Tomaras, B. F. Haynes, D. A. Lauffenburger, C. Bailey-Kellogg, M. Roederer, and G. Alter. 2018. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med. - PMC - PubMed
-
- Agrati C., Cimini E., Sacchi A., Bordoni V., Gioia C., Casetti R., Turchi F., Tripodi M., Martini F. Activated V gamma 9V delta 2 T cells trigger granulocyte functions via MCP-2 release. J. Immunol. 2009;182:522–529. - PubMed
- Agrati, C., E. Cimini, A. Sacchi, V. Bordoni, C. Gioia, R. Casetti, F. Turchi, M. Tripodi, and F. mMartini. 2009. Activated V gamma 9V delta 2 T cells trigger granulocyte functions via MCP-2 release. J Immunol 182: 522-529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

