Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis
- PMID: 31131061
- PMCID: PMC6525994
- DOI: 10.7150/thno.31884
Synechococcus elongatus PCC7942 secretes extracellular vesicles to accelerate cutaneous wound healing by promoting angiogenesis
Abstract
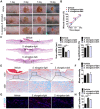
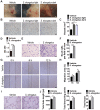
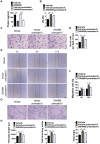
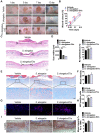
Poor wound healing affects millions of people worldwide each year and needs better therapeutic strategies. Synechococcus elongatus PCC 7942 is a naturally occurring photoautotrophic cyanobacterium that can be easily obtained and large-scale expanded. Here, we investigated the therapeutic efficacy of this cyanobacterium in a mouse model of acute burn injury and whether the secretion of extracellular vesicles (EVs), important mediators of cell paracrine activity, is a key mechanism of the cyanobacterium-induced regulation of wound healing. Methods: The effects of Synechococcus elongatus PCC 7942 on burn wound healing in mice under light or dark conditions were evaluated by measuring wound closure rates, histological and immunofluorescence analyses. A series of assays in vivo and in vitro were conducted to assess the impact of the cyanobacterium on angiogenesis. GW4869 was used to interfere with the secretion of EVs by the cyanobacterium and the abilities of the GW4869-pretreated and untreated Synechococcus elongatus PCC 7942 to regulate endothelial angiogenesis were compared. The direct effects of the cyanobacterium-derived EVs (S. elongatus-EVs) on angiogenesis, wound healing and expressions of a class of pro-inflammatory factors that have regulatory roles in wound healing were also examined. Results: Synechococcus elongatus PCC 7942 treatment under light and dark conditions both significantly promoted angiogenesis and burn wound repair in mice. In vitro, the cyanobacterium enhanced angiogenic activities of endothelial cells, but the effects were markedly blocked by GW4869 pretreatment. S. elongatus-EVs were capable of augmenting endothelial angiogenesis in vitro, and stimulating new blood vessel formation and burn wound healing in mice. The expression of interleukin 6 (IL-6), which has an essential role in angiogenesis during skin wound repair, was induced in wound tissues and wound healing-related cells by S. elongatus-EVs and Synechococcus elongatus PCC 7942. Conclusion: Synechococcus elongatus PCC 7942 has the potential as a promising strategy for therapeutic angiogenesis and wound healing primarily by the delivery of functional EVs, not by its photosynthetic activity. The promotion of IL-6 expression may be a mechanism of the cyanobacterium and its EVs-induced pro-angiogenic and -wound healing effects.
Keywords: Synechococcus elongatus PCC 7942; angiogenesis; extracellular vesicles; wound healing.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis.Theranostics. 2018 Feb 7;8(6):1607-1623. doi: 10.7150/thno.22958. eCollection 2018. Theranostics. 2018. PMID: 29556344 Free PMC article.
-
Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode.J Dermatol Sci. 2018 Feb;89(2):181-190. doi: 10.1016/j.jdermsci.2017.11.008. Epub 2017 Nov 26. J Dermatol Sci. 2018. PMID: 29191392
-
Lactobacillus rhamnosus GG-derived extracellular vesicles promote wound healing via miR-21-5p-mediated re-epithelization and angiogenesis.J Nanobiotechnology. 2024 Oct 19;22(1):644. doi: 10.1186/s12951-024-02893-8. J Nanobiotechnology. 2024. PMID: 39427198 Free PMC article.
-
Extracellular Vesicles Derived from Mesenchymal Stem Cells Promote Wound Healing and Skin Regeneration by Modulating Multiple Cellular Changes: A Brief Review.Genes (Basel). 2023 Jul 25;14(8):1516. doi: 10.3390/genes14081516. Genes (Basel). 2023. PMID: 37628568 Free PMC article. Review.
-
Development of pro-angiogenic skin substitutes for wound healing.Wound Repair Regen. 2024 May-Jun;32(3):208-216. doi: 10.1111/wrr.13154. Epub 2024 Feb 3. Wound Repair Regen. 2024. PMID: 38308588 Review.
Cited by
-
Fructose-coated Angstrom silver inhibits osteosarcoma growth and metastasis via promoting ROS-dependent apoptosis through the alteration of glucose metabolism by inhibiting PDK.Theranostics. 2020 Jun 19;10(17):7710-7729. doi: 10.7150/thno.45858. eCollection 2020. Theranostics. 2020. PMID: 32685015 Free PMC article.
-
Extracellular Vesicle-Mediated Secretion of Protochlorophyllide in the Cyanobacterium Leptolyngbya boryana.Plants (Basel). 2022 Mar 29;11(7):910. doi: 10.3390/plants11070910. Plants (Basel). 2022. PMID: 35406890 Free PMC article.
-
The role of Clec11a in bone construction and remodeling.Front Endocrinol (Lausanne). 2024 Aug 12;15:1429567. doi: 10.3389/fendo.2024.1429567. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39188913 Free PMC article. Review.
-
Glucocorticoid-induced loss of beneficial gut bacterial extracellular vesicles is associated with the pathogenesis of osteonecrosis.Sci Adv. 2022 Apr 15;8(15):eabg8335. doi: 10.1126/sciadv.abg8335. Epub 2022 Apr 13. Sci Adv. 2022. PMID: 35417243 Free PMC article.
-
Cyanobacteria-Based Bio-Oxygen Pump Promoting Hypoxia-Resistant Photodynamic Therapy.Front Bioeng Biotechnol. 2020 Mar 24;8:237. doi: 10.3389/fbioe.2020.00237. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32266251 Free PMC article.
References
-
- He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–9. - PubMed
-
- Li WW, Talcott KE, Zhai AW, Kruger EA, Li VW. The role of therapeutic angiogenesis in tissue repair and regeneration. Adv Skin Wound Care. 2005;18:491–500. quiz 1-2. - PubMed
-
- Atsumi S, Higashide W, Liao JC. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat Biotechnol. 2009;27:1177–80. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

