Intrathecal TRPM8 blocking attenuates cold hyperalgesia via PKC and NF-κB signaling in the dorsal root ganglion of rats with neuropathic pain
- PMID: 31114308
- PMCID: PMC6497852
- DOI: 10.2147/JPR.S197168
Intrathecal TRPM8 blocking attenuates cold hyperalgesia via PKC and NF-κB signaling in the dorsal root ganglion of rats with neuropathic pain
Abstract
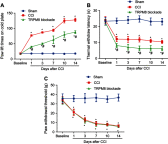
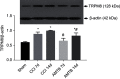
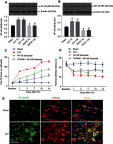
Background: TRPM8 channel plays central roles in the sensitization of nociceptive transduction and is thought as one of the potential targets for the treatment of neuropathic pain. However, the specific molecular mechanisms are still less clear. Methods: Sciatic chronic constriction injury (CCI) rats were intrathecally administered with AMTB (TRPM8-selective antagonist) or PDTC (nuclear factor-kappa B (NF-κB) inhibitor). Cold-, thermal- and mechanical-pain thresholds were examined in CCI and sham-operated rats before and after intrathecal administration of AMTB or PDTC. Protein expression levels of TRPM8 and NF-κB p65, p-PKC/PKC value and p-PKA/PKA value in the CCI ipsilateral L4-6 dorsal root ganglions (DRGs) were analyzed. In addition, the co-expression of TRPM8 and NF-κB was evaluated in DRG. Results: Intrathecal injection of AMTB decreased the cold hypersensitivity and aggravated the thermal-hyperalgesia in the next 2 weeks after CCI surgery. The protein expression of TRPM8 and NF-κB p65 in the ipsilateral DRGs significantly increased after CCI surgery, which can be reversed by intrathecal administration of AMTB. The PKC, PKA, p-PKC/PKC and p-PKA/PKA values showed significantly increase after CCI surgery, while intrathecal AMTB administration offset the expression increase of PKC, p-PKC and p-PKC/PKC but PKA or p-PKA/PKA in the DRG. NF-κB inhibitor not only efficiently increased the cold-, thermal-pain threshold of CCI rats, but also enhanced AMTB's anti-cold pain effect although exerted no anti-thermal hyperalgesia effect compared with TRPM8 blockade group. Immunofluorescence results showed co-expression of TRPM8 and NF-κB in DRG neurons. Conclusion: TRPM8 channels in DRGs participate in the pathogenesis of cold and thermal hyperalgesia (not mechanical allodynia) in rats with neuropathic pain, which could be regulated by PKC (not PKA) and NF-κB signaling. TRPM8 channel, PKC and NF-κB are potential targets for cold hyperalgesia treatment in neuropathic pain patients.
Keywords: NF-κB; PKC; TRPM8; dorsal root ganglia; neuropathic pain.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Effect of PKC/NF-κB on the Regulation of P2X3 Receptor in Dorsal Root Ganglion in Rats with Sciatic Nerve Injury.Pain Res Manag. 2020 Sep 17;2020:7104392. doi: 10.1155/2020/7104392. eCollection 2020. Pain Res Manag. 2020. PMID: 33014214 Free PMC article.
-
Downregulations of TRPM8 expression and membrane trafficking in dorsal root ganglion mediate the attenuation of cold hyperalgesia in CCI rats induced by GFRα3 knockdown.Brain Res Bull. 2017 Oct;135:8-24. doi: 10.1016/j.brainresbull.2017.08.002. Epub 2017 Sep 1. Brain Res Bull. 2017. PMID: 28867384
-
Role of TRPM8 in dorsal root ganglion in nerve injury-induced chronic pain.BMC Neurosci. 2011 Nov 23;12:120. doi: 10.1186/1471-2202-12-120. BMC Neurosci. 2011. PMID: 22111979 Free PMC article.
-
Implications of intrathecal pertussis toxin animal model on the cellular mechanisms of neuropathic pain syndrome.Acta Anaesthesiol Sin. 2003 Dec;41(4):187-96. Acta Anaesthesiol Sin. 2003. PMID: 14768516 Review.
-
The cool things to know about TRPM8!Channels (Austin). 2020 Dec;14(1):413-420. doi: 10.1080/19336950.2020.1841419. Channels (Austin). 2020. PMID: 33147416 Free PMC article. Review.
Cited by
-
Hypoxia-Mediated Complement 1q Binding Protein Regulates Metastasis and Chemoresistance in Triple-Negative Breast Cancer and Modulates the PKC-NF-κB-VCAM-1 Signaling Pathway.Front Cell Dev Biol. 2021 Feb 23;9:607142. doi: 10.3389/fcell.2021.607142. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33708767 Free PMC article.
-
Antinociceptive Effects of Aaptamine, a Sponge Component, on Peripheral Neuropathy in Rats.Mar Drugs. 2023 Feb 4;21(2):113. doi: 10.3390/md21020113. Mar Drugs. 2023. PMID: 36827154 Free PMC article.
-
Suppression of TRPV1/TRPM8/P2Y Nociceptors by Withametelin via Downregulating MAPK Signaling in Mouse Model of Vincristine-Induced Neuropathic Pain.Int J Mol Sci. 2021 Jun 4;22(11):6084. doi: 10.3390/ijms22116084. Int J Mol Sci. 2021. PMID: 34199936 Free PMC article.
-
Effect of PKC/NF-κB on the Regulation of P2X3 Receptor in Dorsal Root Ganglion in Rats with Sciatic Nerve Injury.Pain Res Manag. 2020 Sep 17;2020:7104392. doi: 10.1155/2020/7104392. eCollection 2020. Pain Res Manag. 2020. PMID: 33014214 Free PMC article.
-
Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation.Front Immunol. 2020 Oct 23;11:590261. doi: 10.3389/fimmu.2020.590261. eCollection 2020. Front Immunol. 2020. PMID: 33193423 Free PMC article. Review.
References
-
- Meacham K, Shepherd A. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep. 2017;21(6):28. - PubMed
LinkOut - more resources
Full Text Sources

