Prospective Assessment of Cytomegalovirus Immunity in High-Risk Donor-Seropositive/Recipient-Seronegative Liver Transplant Recipients Receiving Either Preemptive Therapy or Antiviral Prophylaxis
- PMID: 31112280
- PMCID: PMC7325329
- DOI: 10.1093/infdis/jiz181
Prospective Assessment of Cytomegalovirus Immunity in High-Risk Donor-Seropositive/Recipient-Seronegative Liver Transplant Recipients Receiving Either Preemptive Therapy or Antiviral Prophylaxis
Abstract
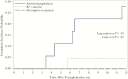
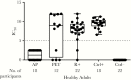
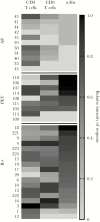
The differential impact of preemptive therapy (PET) and antiviral prophylaxis (AP) on development of cytomegalovirus (CMV)-specific neutralizing antibody (nAb) and T-cell responses have not previously been directly compared in high-risk donor-seropositive/recipient-seronegative (D+R-) organ transplant recipients. We prospectively assessed T-cell and nAb responses 3 months after transplantation in cohorts of high-risk D+R- liver transplant recipients who received either PET (n = 15) or AP (n = 25) and a control group of CMV-seropositive transplant recipients (R+) (AP; n = 24). CMV phosphoprotein 65 (pp65)- and immediate early protein 1-specific multifunctional T-cell responses were determined by means of intracellular cytokine staining and nAbs against BADrUL131-Y4 CMV in adult retinal pigment epithelial cell line-19 human epithelial cells; nAbs were detected in 8 of 12 (67%) in the PET group, none of 17 in the AP group, and 20 of 22 (91%) in the R+ group. Multifunctional CD8 and CD4 T-cell responses to pp65 were generally similar between PET and R+ groups, and lower for the AP group; multifunctional CD4 responses were similar across all groups. Among D+R- liver transplant recipients, PET was associated with the development of greater nAb and multifunctional CD8 T-cell responses compared with AP, providing a potential mechanism to explain the relative protection against late-onset disease with PET. Future studies are needed to define specific immune parameters predictive of late-onset CMV disease with AP.
Keywords: Cytomegalovirus; immunity; transplant.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Cytomegalovirus immunity in high-risk liver transplant recipients following preemptive antiviral therapy versus prophylaxis.JCI Insight. 2024 Sep 24;9(18):e180115. doi: 10.1172/jci.insight.180115. JCI Insight. 2024. PMID: 39099206 Free PMC article. Clinical Trial.
-
Comparison of Preemptive Therapy and Antiviral Prophylaxis for Prevention of Cytomegalovirus in Seropositive Liver Transplant Recipients.Transplantation. 2018 Apr;102(4):632-639. doi: 10.1097/TP.0000000000002029. Transplantation. 2018. PMID: 29215460
-
Effect of Preemptive Therapy vs Antiviral Prophylaxis on Cytomegalovirus Disease in Seronegative Liver Transplant Recipients With Seropositive Donors: A Randomized Clinical Trial.JAMA. 2020 Apr 14;323(14):1378-1387. doi: 10.1001/jama.2020.3138. JAMA. 2020. PMID: 32286644 Free PMC article. Clinical Trial.
-
Prevention and treatment of cytomegalovirus infection in organ transplant recipients.Transpl Infect Dis. 1999 Sep;1(3):187-203. doi: 10.1034/j.1399-3062.1999.010307.x. Transpl Infect Dis. 1999. PMID: 11428989 Review.
-
Ganciclovir. An update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients.Drugs. 1998 Jul;56(1):115-46. doi: 10.2165/00003495-199856010-00012. Drugs. 1998. PMID: 9664203 Review.
Cited by
-
Unexpected Cytomegalovirus (CMV) Replication Kinetics in CMV Donor-Seropositive, Recipient-Seronegative Liver Transplant Recipients Receiving Preemptive Antiviral Therapy.J Infect Dis. 2022 Feb 1;225(3):436-442. doi: 10.1093/infdis/jiab132. J Infect Dis. 2022. PMID: 33755176 Free PMC article.
-
Cytomegalovirus immunity in high-risk liver transplant recipients following preemptive antiviral therapy versus prophylaxis.JCI Insight. 2024 Sep 24;9(18):e180115. doi: 10.1172/jci.insight.180115. JCI Insight. 2024. PMID: 39099206 Free PMC article. Clinical Trial.
-
Prevention and management of CMV infection in pediatric solid organ transplant recipients.Front Pediatr. 2023 Feb 20;11:1098434. doi: 10.3389/fped.2023.1098434. eCollection 2023. Front Pediatr. 2023. PMID: 36891229 Free PMC article. Review.
-
A Review of Murine Cytomegalovirus as a Model for Human Cytomegalovirus Disease-Do Mice Lie?Int J Mol Sci. 2020 Dec 28;22(1):214. doi: 10.3390/ijms22010214. Int J Mol Sci. 2020. PMID: 33379272 Free PMC article. Review.
-
Progress and Challenges in the Prevention, Diagnosis, and Management of Cytomegalovirus Infection in Transplantation.Clin Microbiol Rev. 2020 Oct 28;34(1):e00043-19. doi: 10.1128/CMR.00043-19. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33115722 Free PMC article. Review.
References
-
- Mattes FM, Vargas A, Kopycinski J, et al. . Functional impairment of cytomegalovirus specific CD8 T cells predicts high-level replication after renal transplantation. Am J Transplant 2008; 8:990–9. - PubMed
-
- Nebbia G, Mattes FM, Smith C, et al. . Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant 2008; 8:2590–9. - PubMed
-
- Gerna G, Revello MG, Baldanti F, Percivalle E, Lilleri D. The pentameric complex of human cytomegalovirus: cell tropism, virus dissemination, immune response and vaccine development. J Gen Virol 2017; 98:2215–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

