Kaposi Sarcoma-Associated Herpesvirus and Staphylococcus aureus Coinfection in Oral Cavities of HIV-Positive Patients: A Unique Niche for Oncogenic Virus Lytic Reactivation
- PMID: 31111897
- PMCID: PMC7325796
- DOI: 10.1093/infdis/jiz249
Kaposi Sarcoma-Associated Herpesvirus and Staphylococcus aureus Coinfection in Oral Cavities of HIV-Positive Patients: A Unique Niche for Oncogenic Virus Lytic Reactivation
Abstract
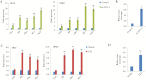
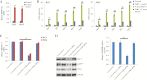
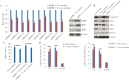
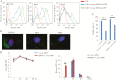
Collectively, viruses are the principal cause of cancers arising in patients with immune dysfunction, including human immunodeficiency virus (HIV)-positive patients. Kaposi sarcoma (KS) etiologically linked to Kaposi sarcoma-associated herpesvirus (KSHV) continues to be the most common AIDS-associated tumor. The involvement of the oral cavity represents one of the most common clinical manifestations of this tumor. HIV infection incurs an increased risk among individuals with periodontal diseases and oral carriage of a variety of pathogenic bacteria. However, whether interactions involving periodontal bacteria and oncogenic viruses in the local environment facilitate replication or maintenance of these viruses in the oral cavity of HIV-positive patients remain largely unknown. We previously showed that pathogen-associated molecular patterns (PAMPs) from specific periodontal bacteria promoted KSHV entry into oral cells and subsequent establishment of latency. In the current study, we demonstrate that Staphylococcus aureus, one of common pathogens causing infection in HIV-positive patients, and its PAMPs can effectively induce KSHV lytic reactivation from infected oral cells, through the Toll-like receptor reactive oxygen species and cyclin D1-Dicer-viral microRNA axis. This investigation provides further clinical evidence about the relevance of coinfection due to these 2 pathogens in the oral cavities of a cohort HIV-positive patients and reveals novel mechanisms through which these coinfecting pathogens potentially promote virus-associated cancer development in the unique niche of immunocompromised patients.
Keywords: Staphylococcus aureus; HIV; KSHV; Kaposi sarcoma; microRNA.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Are There Clues to Oral Kaposi Sarcoma-Associated Herpesvirus Shedding and Kaposi Sarcoma Oncogenesis in the Oral Microbiome?J Infect Dis. 2020 Mar 28;221(8):1226-1228. doi: 10.1093/infdis/jiz250. J Infect Dis. 2020. PMID: 31111901 Free PMC article. No abstract available.
Similar articles
-
Porphyromonas gingivalis coinfects with KSHV in oral cavities of HIV+ patients and induces viral lytic reactivation.J Med Virol. 2020 Dec;92(12):3862-3867. doi: 10.1002/jmv.26028. Epub 2020 Jun 2. J Med Virol. 2020. PMID: 32436999 Free PMC article.
-
Lipoteichoic acid (LTA) and lipopolysaccharides (LPS) from periodontal pathogenic bacteria facilitate oncogenic herpesvirus infection within primary oral cells.PLoS One. 2014 Jun 27;9(6):e101326. doi: 10.1371/journal.pone.0101326. eCollection 2014. PLoS One. 2014. PMID: 24971655 Free PMC article.
-
Signatures of oral microbiome in HIV-infected individuals with oral Kaposi's sarcoma and cell-associated KSHV DNA.PLoS Pathog. 2020 Jan 17;16(1):e1008114. doi: 10.1371/journal.ppat.1008114. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31951641 Free PMC article. Clinical Trial.
-
Infection of KSHV and Interaction with HIV: The Bad Romance.Adv Exp Med Biol. 2017;1018:237-251. doi: 10.1007/978-981-10-5765-6_15. Adv Exp Med Biol. 2017. PMID: 29052142 Review.
-
Regulation of KSHV Latency and Lytic Reactivation.Viruses. 2020 Sep 17;12(9):1034. doi: 10.3390/v12091034. Viruses. 2020. PMID: 32957532 Free PMC article. Review.
Cited by
-
The Role of Bacteria in KSHV Infection and KSHV-Induced Cancers.Cancers (Basel). 2021 Aug 25;13(17):4269. doi: 10.3390/cancers13174269. Cancers (Basel). 2021. PMID: 34503079 Free PMC article. Review.
-
Unusual Localization of AIDS-Related Kaposi's Sarcoma in a Heterosexual Male during the COVID-19 Pandemic: A Case Report.Trop Med Infect Dis. 2024 Feb 13;9(2):47. doi: 10.3390/tropicalmed9020047. Trop Med Infect Dis. 2024. PMID: 38393136 Free PMC article.
-
Oral Kaposi Sarcoma in two patients living with HIV despite sustained viral suppression: New clues.J Clin Exp Dent. 2022 May 1;14(5):e453-e456. doi: 10.4317/jced.59610. eCollection 2022 May. J Clin Exp Dent. 2022. PMID: 35582353 Free PMC article.
-
Anti-viral and pro-inflammatory functions of Toll-like receptors during gamma-herpesvirus infections.Virol J. 2021 Nov 8;18(1):218. doi: 10.1186/s12985-021-01678-x. Virol J. 2021. PMID: 34749760 Free PMC article. Review.
-
Stress-Related Herpesvirus Reactivation in Badgers Can Result in Clostridium Proliferation.Ecohealth. 2021 Dec;18(4):440-450. doi: 10.1007/s10393-021-01568-2. Epub 2021 Dec 6. Ecohealth. 2021. PMID: 34870778 Free PMC article.
References
-
- Vanni T, Sprinz E, Machado MW, Santana Rde C, Fonseca BA, Schwartsmann G. Systemic treatment of AIDS-related Kaposi sarcoma: current status and perspectives. Cancer Treat Rev 2006; 32:445–55. - PubMed
-
- Engels EA, Biggar RJ, Hall HI, et al. . Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008; 123:187–94. - PubMed
-
- Bonnet F, Lewden C, May T, et al. . Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer 2004; 101:317–24. - PubMed
-
- Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med 2007; 357:1352–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

