p53 at the Crossroads between Different Types of HDAC Inhibitor-Mediated Cancer Cell Death
- PMID: 31096697
- PMCID: PMC6567317
- DOI: 10.3390/ijms20102415
p53 at the Crossroads between Different Types of HDAC Inhibitor-Mediated Cancer Cell Death
Abstract
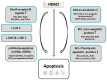
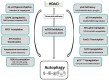
Cancer is a complex genetic and epigenetic-based disease that has developed an armada of mechanisms to escape cell death. The deregulation of apoptosis and autophagy, which are basic processes essential for normal cellular activity, are commonly encountered during the development of human tumors. In order to assist the cancer cell in defeating the imbalance between cell growth and cell death, histone deacetylase inhibitors (HDACi) have been employed to reverse epigenetically deregulated gene expression caused by aberrant post-translational protein modifications. These interfere with histone acetyltransferase- and deacetylase-mediated acetylation of both histone and non-histone proteins, and thereby exert a wide array of HDACi-stimulated cytotoxic effects. Key determinants of HDACi lethality that interfere with cellular growth in a multitude of tumor cells are apoptosis and autophagy, which are either mutually exclusive or activated in combination. Here, we compile known molecular signals and pathways involved in the HDACi-triggered induction of apoptosis and autophagy. Currently, the factors that determine the mode of HDACi-elicited cell death are mostly unclear. Correspondingly, we also summarized as yet established intertwined mechanisms, in particular with respect to the oncogenic tumor suppressor protein p53, that drive the interplay between apoptosis and autophagy in response to HDACi. In this context, we also note the significance to determine the presence of functional p53 protein levels in the cancer cell. The confirmation of the context-dependent function of autophagy will pave the way to improve the benefit from HDACi-mediated cancer treatment.
Keywords: HDAC; HDACi; SAHA; apoptosis; autophagy; cancer; cell death; p53; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Epigenetic Targeting of Autophagy via HDAC Inhibition in Tumor Cells: Role of p53.Int J Mol Sci. 2018 Dec 8;19(12):3952. doi: 10.3390/ijms19123952. Int J Mol Sci. 2018. PMID: 30544838 Free PMC article. Review.
-
Histone Deacetylase Inhibitor-Induced Autophagy in Tumor Cells: Implications for p53.Int J Mol Sci. 2017 Aug 31;18(9):1883. doi: 10.3390/ijms18091883. Int J Mol Sci. 2017. PMID: 30563957 Free PMC article. Review.
-
Novel hydroxamic acid derivative induces apoptosis and constrains autophagy in leukemic cells.J Adv Res. 2024 Jun;60:201-214. doi: 10.1016/j.jare.2023.07.005. Epub 2023 Jul 17. J Adv Res. 2024. PMID: 37467961 Free PMC article.
-
Histone deacetylase inhibitors VPA and TSA induce apoptosis and autophagy in pancreatic cancer cells.Cell Oncol (Dordr). 2017 Apr;40(2):167-180. doi: 10.1007/s13402-017-0314-z. Epub 2017 Feb 3. Cell Oncol (Dordr). 2017. PMID: 28160167
-
Targeting Histone Deacetylases with Natural and Synthetic Agents: An Emerging Anticancer Strategy.Nutrients. 2018 Jun 6;10(6):731. doi: 10.3390/nu10060731. Nutrients. 2018. PMID: 29882797 Free PMC article. Review.
Cited by
-
Mefloquine Inhibits Esophageal Squamous Cell Carcinoma Tumor Growth by Inducing Mitochondrial Autophagy.Front Oncol. 2020 Jul 28;10:1217. doi: 10.3389/fonc.2020.01217. eCollection 2020. Front Oncol. 2020. PMID: 32850358 Free PMC article.
-
HDACs/mTOR inhibitor synergizes with pyrotinib in HER2-positive pancreatic cancer through degradation of mutant P53.Cancer Cell Int. 2022 Dec 1;22(1):380. doi: 10.1186/s12935-022-02807-4. Cancer Cell Int. 2022. PMID: 36457011 Free PMC article.
-
Anticancer Therapy with HDAC Inhibitors: Mechanism-Based Combination Strategies and Future Perspectives.Cancers (Basel). 2021 Feb 5;13(4):634. doi: 10.3390/cancers13040634. Cancers (Basel). 2021. PMID: 33562653 Free PMC article. Review.
-
Pancreatic Neuroendocrine Tumors: Signaling Pathways and Epigenetic Regulation.Int J Mol Sci. 2024 Jan 22;25(2):1331. doi: 10.3390/ijms25021331. Int J Mol Sci. 2024. PMID: 38279330 Free PMC article. Review.
-
CREB1 regulates RECQL4 to inhibit mitophagy and promote esophageal cancer metastasis.J Clin Biochem Nutr. 2024 Sep;75(2):102-110. doi: 10.3164/jcbn.23-118. Epub 2024 Mar 22. J Clin Biochem Nutr. 2024. PMID: 39345293 Free PMC article.
References
-
- Lockshin R., Williams C. Programmed cell death—II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J. Insect Physiol. 1964;10:643–649. doi: 10.1016/0022-1910(64)90034-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

