Influenza epidemiology and influenza vaccine effectiveness during the 2015-2016 season: results from the Global Influenza Hospital Surveillance Network
- PMID: 31088481
- PMCID: PMC6518734
- DOI: 10.1186/s12879-019-4017-0
Influenza epidemiology and influenza vaccine effectiveness during the 2015-2016 season: results from the Global Influenza Hospital Surveillance Network
Abstract
Background: The Global Influenza Hospital Surveillance Network is an international platform whose primary objective is to study severe cases of influenza requiring hospitalization.
Methods: During the 2015-2016 influenza season, 11 sites in the Global Influenza Hospital Surveillance Network in nine countries (Russian Federation, Czech Republic, Turkey, France, China, Spain, Mexico, India, and Brazil) participated in a prospective, active-surveillance, hospital-based epidemiological study. Influenza infection was confirmed by reverse transcription-polymerase chain reaction. Influenza vaccine effectiveness (IVE) against laboratory-confirmed influenza was estimated using a test-negative approach.
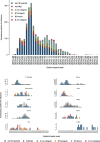
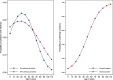
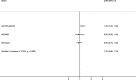
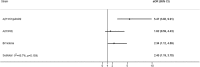
Results: 9882 patients with laboratory results were included of which 2415 (24.4%) were positive for influenza, including 1415 (14.3%) for A(H1N1)pdm09, 235 (2.4%) for A(H3N2), 180 (1.8%) for A not subtyped, 45 (0.5%) for B/Yamagata-lineage, 532 (5.4%) for B/Victoria-lineage, and 33 (0.3%) for B not subtyped. Of included admissions, 39% were < 5 years of age and 67% had no underlying conditions. The odds of being admitted with influenza were higher among pregnant than non-pregnant women (odds ratio, 2.82 [95% confidence interval (CI), 1.90 to 4.19]). Adjusted IVE against influenza-related hospitalization was 16.3% (95% CI, 0.4 to 29.7). Among patients targeted for influenza vaccination, adjusted IVE against hospital admission with influenza was 16.2% (95% CI, - 3.6 to 32.2) overall, 23.0% (95% CI, - 3.3 to 42.6) against A(H1N1)pdm09, and - 25.6% (95% CI, - 86.3 to 15.4) against B/Victoria lineage.
Conclusions: The 2015-2016 influenza season was dominated by A(H1N1)pdm09 and B/Victoria-lineage. Hospitalization with influenza often occurred in healthy and young individuals, and pregnant women were at increased risk of influenza-related hospitalization. Influenza vaccines provided low to moderate protection against hospitalization with influenza and no protection against the predominant circulating B lineage, highlighting the need for more effective and broader influenza vaccines.
Keywords: Epidemiological study; Hospitalization; Influenza; Surveillance; Vaccine; Virus.
Conflict of interest statement
Ethics approval and consent to participate
The GIHSN study protocol was approved by the institutional review board of each participating site: in India, the Institutional Ethics Committee of the Sher-i-Kashmir Institute of Medical Sciences, Srinagar; in Beijing, China, “The Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster”; in France, “CPP Ile de France IV, Saint-Louis hospital in Paris”; in the Czech Republic, the “Ethics Committee of the Hospital Na Bulovce”; in Turkey, the “Hacettepe University Non-interventional Clinical Research Ethics Board”; in Moscow, Russia, “The local Ethic Committee of Hospital #1 for Infectious Diseases of Moscow Health Department”; in Brazil (Fortaleza & Curitiba), the “Ethical Committee of the Evandro Chagas National Institute of Infectology (INI-Fiocruz)”; in Mexico, the “Research Ethics Committee of the National Institute of Medical Science and Nutrition Salvador Zubirán” & “Research Committee of the National Institute of Medical Science and Nutrition Salvador Zubirán”; in St Petersburg, Russia, “Local Ethical Committee under the FGBU ‘Research Institute of Influenza’ of the Ministry of Health of the Russian Federation”; and in Spain, the “Research Ethics Committee of the General Directorate of Public Health and High Center for Public Health Research (CEI DGSP-CSISP)”. All subjects or legal representatives provided written or (if approved by the associated institutional review board) oral informed consent; consent to participate was collected from the parents/guardians of any participants under 16 years of age.
Consent for publication
Not applicable.
Competing interests
BJC received grants from Sanofi Pasteur unrelated to the submitted work. PK has served as a consultant to Sanofi, Zydus, and Cadila. All other authors declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


Similar articles
-
Low influenza vaccine effectiveness and the effect of previous vaccination in preventing admission with A(H1N1)pdm09 or B/Victoria-Lineage in patients 60 years old or older during the 2015/2016 influenza season.Vaccine. 2017 Dec 19;35(52):7331-7338. doi: 10.1016/j.vaccine.2017.10.100. Epub 2017 Nov 8. Vaccine. 2017. PMID: 29128380
-
Influenza epidemiology and influenza vaccine effectiveness during the 2016-2017 season in the Global Influenza Hospital Surveillance Network (GIHSN).BMC Public Health. 2019 May 2;19(1):487. doi: 10.1186/s12889-019-6713-5. BMC Public Health. 2019. PMID: 31046725 Free PMC article.
-
2012-2013 Seasonal influenza vaccine effectiveness against influenza hospitalizations: results from the global influenza hospital surveillance network.PLoS One. 2014 Jun 19;9(6):e100497. doi: 10.1371/journal.pone.0100497. eCollection 2014. PLoS One. 2014. PMID: 24945510 Free PMC article.
-
Influenza vaccine effectiveness against laboratory-confirmed influenza in hospitalised adults aged 60 years or older, Valencia Region, Spain, 2017/18 influenza season.Euro Surveill. 2019 Aug;24(31):1800461. doi: 10.2807/1560-7917.ES.2019.24.31.1800461. Euro Surveill. 2019. PMID: 31387672 Free PMC article.
-
2012/13 influenza vaccine effectiveness against hospitalised influenza A(H1N1)pdm09, A(H3N2) and B: estimates from a European network of hospitals.Euro Surveill. 2015 Jan 15;20(2):21011. doi: 10.2807/1560-7917.es2015.20.2.21011. Euro Surveill. 2015. PMID: 25613779
Cited by
-
Age-Specific Etiology of Severe Acute Respiratory Infections and Influenza Vaccine Effectivity in Prevention of Hospitalization in Russia, 2018-2019 Season.J Epidemiol Glob Health. 2021 Dec;11(4):413-425. doi: 10.1007/s44197-021-00009-1. Epub 2021 Oct 28. J Epidemiol Glob Health. 2021. PMID: 34734387 Free PMC article.
-
Clinical characteristics and outcomes of influenza A and B virus infection in adult Australian hospitalised patients.BMC Infect Dis. 2020 Dec 1;20(1):913. doi: 10.1186/s12879-020-05670-8. BMC Infect Dis. 2020. PMID: 33261559 Free PMC article.
-
Characteristics and evolution of hemagglutinin and neuraminidase genes of Influenza A(H3N2) viruses in Thailand during 2015 to 2018.PeerJ. 2024 Jun 3;12:e17523. doi: 10.7717/peerj.17523. eCollection 2024. PeerJ. 2024. PMID: 38846750 Free PMC article.
-
Age Differences in Comorbidities, Presenting Symptoms, and Outcomes of Influenza Illness Requiring Hospitalization: A Worldwide Perspective From the Global Influenza Hospital Surveillance Network.Open Forum Infect Dis. 2023 May 7;10(6):ofad244. doi: 10.1093/ofid/ofad244. eCollection 2023 Jun. Open Forum Infect Dis. 2023. PMID: 37383245 Free PMC article.
-
Predictors for influenza vaccination among Thai pregnant woman: The role of physicians in increasing vaccine uptake.Influenza Other Respir Viruses. 2019 Nov;13(6):582-592. doi: 10.1111/irv.12674. Epub 2019 Aug 16. Influenza Other Respir Viruses. 2019. PMID: 31419068 Free PMC article.
References
-
- Puig-Barberà Joan, Tormos Anita, Trushakova Svetlana, Sominina Anna, Pisareva Maria, Ciblak Meral A., Badur Selim, Yu Hongjie, Cowling Benjamin J., Burtseva Elena. The Global Influenza Hospital Surveillance Network (GIHSN): a new platform to describe the epidemiology of severe influenza. Influenza and Other Respiratory Viruses. 2015;9(6):277–286. doi: 10.1111/irv.12335. - DOI - PMC - PubMed
-
- Puig-Barbera J, Natividad-Sancho A, Launay O, Burtseva E, Ciblak MA, Tormos A, et al. 2012-2013 seasonal influenza vaccine effectiveness against influenza hospitalizations: results from the Global Influenza Hospital Surveillance Network. PLoS One. 2014;9(6):e100497. doi: 10.1371/journal.pone.0100497. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

