Comprehensive knockout analysis of the Rab family GTPases in epithelial cells
- PMID: 31072826
- PMCID: PMC6548125
- DOI: 10.1083/jcb.201810134
Comprehensive knockout analysis of the Rab family GTPases in epithelial cells
Abstract
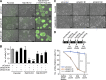
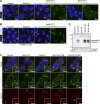
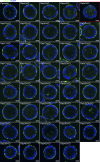
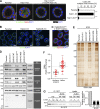
The Rab family of small GTPases comprises the largest number of proteins (∼60 in mammals) among the regulators of intracellular membrane trafficking, but the precise function of many Rabs and the functional redundancy and diversity of Rabs remain largely unknown. Here, we generated a comprehensive collection of knockout (KO) MDCK cells for the entire Rab family. We knocked out closely related paralogs simultaneously (Rab subfamily knockout) to circumvent functional compensation and found that Rab1A/B and Rab5A/B/C are critical for cell survival and/or growth. In addition, we demonstrated that Rab6-KO cells lack the basement membrane, likely because of the inability to secrete extracellular matrix components. Further analysis revealed the general requirement of Rab6 for secretion of soluble cargos. Transport of transmembrane cargos to the plasma membrane was also significantly delayed in Rab6-KO cells, but the phenotype was relatively mild. Our Rab-KO collection, which shares the same background, would be a valuable resource for analyzing a variety of membrane trafficking events.
© 2019 Homma et al.
Figures
Similar articles
-
Methods for Establishing Rab Knockout MDCK Cells.Methods Mol Biol. 2021;2293:243-256. doi: 10.1007/978-1-0716-1346-7_17. Methods Mol Biol. 2021. PMID: 34453722
-
A comprehensive analysis of Rab GTPases reveals a role for Rab34 in serum starvation-induced primary ciliogenesis.J Biol Chem. 2020 Sep 4;295(36):12674-12685. doi: 10.1074/jbc.RA119.012233. Epub 2020 Jul 15. J Biol Chem. 2020. PMID: 32669361 Free PMC article.
-
Regulation of podocalyxin trafficking by Rab small GTPases in 2D and 3D epithelial cell cultures.J Cell Biol. 2016 May 9;213(3):355-69. doi: 10.1083/jcb.201512024. Epub 2016 May 2. J Cell Biol. 2016. PMID: 27138252 Free PMC article.
-
How can mammalian Rab small GTPases be comprehensively analyzed?: Development of new tools to comprehensively analyze mammalian Rabs in membrane traffic.Histol Histopathol. 2010 Nov;25(11):1473-80. doi: 10.14670/HH-25.1473. Histol Histopathol. 2010. PMID: 20865669 Review.
-
Rab GTPases and microtubule motors.Biochem Soc Trans. 2011 Oct;39(5):1202-6. doi: 10.1042/BST0391202. Biochem Soc Trans. 2011. PMID: 21936789 Review.
Cited by
-
The GTPase RAB6 is required for stem cell maintenance and cell migration in the gut epithelium.Development. 2024 Nov 1;151(21):dev203038. doi: 10.1242/dev.203038. Epub 2024 Oct 21. Development. 2024. PMID: 39431301 Free PMC article.
-
Newer Methods Drive Recent Insights into Rab GTPase Biology: An Overview.Methods Mol Biol. 2021;2293:1-18. doi: 10.1007/978-1-0716-1346-7_1. Methods Mol Biol. 2021. PMID: 34453706
-
Retrograde transport of CDMPR depends on several machineries as analyzed by sulfatable nanobodies.Life Sci Alliance. 2022 Mar 21;5(7):e202101269. doi: 10.26508/lsa.202101269. Print 2022 Mar. Life Sci Alliance. 2022. PMID: 35314489 Free PMC article.
-
Crumbs organizes the transport machinery by regulating apical levels of PI(4,5)P2 in Drosophila.Elife. 2019 Nov 7;8:e50900. doi: 10.7554/eLife.50900. Elife. 2019. PMID: 31697234 Free PMC article.
-
Griscelli Syndrome Type 2 Sine Albinism: Unraveling Differential RAB27A Effector Engagement.Front Immunol. 2020 Dec 10;11:612977. doi: 10.3389/fimmu.2020.612977. eCollection 2020. Front Immunol. 2020. PMID: 33362801 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions

