Mechanisms of the Metabolic Shift during Somatic Cell Reprogramming
- PMID: 31067778
- PMCID: PMC6539623
- DOI: 10.3390/ijms20092254
Mechanisms of the Metabolic Shift during Somatic Cell Reprogramming
Abstract
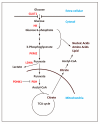
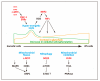
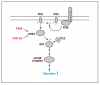
Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), hold a huge promise for regenerative medicine, drug development, and disease modeling. PSCs have unique metabolic features that are akin to those of cancer cells, in which glycolysis predominates to produce energy as well as building blocks for cellular components. Recent studies indicate that the unique metabolism in PSCs is not a mere consequence of their preference for a low oxygen environment, but is an active process for maintaining self-renewal and pluripotency, possibly in preparation for rapid response to the metabolic demands of differentiation. Understanding the regulatory mechanisms of this unique metabolism in PSCs is essential for proper derivation, generation, and maintenance of PSCs. In this review, we discuss the metabolic features of PSCs and describe the current understanding of the mechanisms of the metabolic shift during reprogramming from somatic cells to iPSCs, in which the metabolism switches from oxidative phosphorylation (OxPhos) to glycolysis.
Keywords: ESC; OxPhos: glycolysis; PSC; hypoxia; iPSC; metabolism; mitochondrion; somatic cell reprogramming.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Metabolic remodeling during somatic cell reprogramming to induced pluripotent stem cells: involvement of hypoxia-inducible factor 1.Inflamm Regen. 2020 May 12;40:8. doi: 10.1186/s41232-020-00117-8. eCollection 2020. Inflamm Regen. 2020. PMID: 32426078 Free PMC article. Review.
-
Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal.Cell Stem Cell. 2012 Nov 2;11(5):589-95. doi: 10.1016/j.stem.2012.10.005. Cell Stem Cell. 2012. PMID: 23122286 Free PMC article.
-
Mitochondria and pluripotent stem cells function.Yi Chuan. 2016 Jul 20;38(7):603-611. doi: 10.16288/j.yczz.16-001. Yi Chuan. 2016. PMID: 27733333 Review.
-
Mitochondrial function in pluripotent stem cells and cellular reprogramming.Gerontology. 2014;60(2):174-82. doi: 10.1159/000355050. Epub 2013 Nov 19. Gerontology. 2014. PMID: 24281332 Review.
-
Pluripotent stem cell energy metabolism: an update.EMBO J. 2015 Jan 13;34(2):138-53. doi: 10.15252/embj.201490446. Epub 2014 Dec 4. EMBO J. 2015. PMID: 25476451 Free PMC article. Review.
Cited by
-
Interplay between Metabolism Reprogramming and Epithelial-to-Mesenchymal Transition in Cancer Stem Cells.Cancers (Basel). 2021 Apr 20;13(8):1973. doi: 10.3390/cancers13081973. Cancers (Basel). 2021. PMID: 33923958 Free PMC article. Review.
-
Induced Pluripotency: A Powerful Tool for In Vitro Modeling.Int J Mol Sci. 2020 Nov 24;21(23):8910. doi: 10.3390/ijms21238910. Int J Mol Sci. 2020. PMID: 33255453 Free PMC article. Review.
-
In Vitro Induction of Pluripotency from Equine Fibroblasts in 20% or 5% Oxygen.Stem Cells Int. 2020 Nov 26;2020:8814989. doi: 10.1155/2020/8814989. eCollection 2020. Stem Cells Int. 2020. PMID: 33456472 Free PMC article.
-
PRPS1-mediated purine biosynthesis is critical for pluripotent stem cell survival and stemness.Aging (Albany NY). 2021 Jan 20;13(3):4063-4078. doi: 10.18632/aging.202372. Epub 2021 Jan 20. Aging (Albany NY). 2021. PMID: 33493137 Free PMC article.
-
"Time Is out of Joint" in Pluripotent Stem Cells: How and Why.Int J Mol Sci. 2024 Feb 8;25(4):2063. doi: 10.3390/ijms25042063. Int J Mol Sci. 2024. PMID: 38396740 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

