IgG and Fcγ Receptors in Intestinal Immunity and Inflammation
- PMID: 31031776
- PMCID: PMC6473071
- DOI: 10.3389/fimmu.2019.00805
IgG and Fcγ Receptors in Intestinal Immunity and Inflammation
Abstract
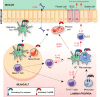
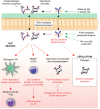
Fcγ receptors (FcγR) are cell surface glycoproteins that mediate cellular effector functions of immunoglobulin G (IgG) antibodies. Genetic variation in FcγR genes can influence susceptibility to a variety of antibody-mediated autoimmune and inflammatory disorders, including systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). More recently, however, genetic studies have implicated altered FcγR signaling in the pathogenesis of inflammatory bowel disease (IBD), a condition classically associated with dysregulated innate and T cell immunity. Specifically, a variant of the activating receptor, FcγRIIA, with low affinity for IgG, confers protection against the development of ulcerative colitis, a subset of IBD, leading to a re-evaluation of the role of IgG and FcγRs in gastrointestinal tract immunity, an organ system traditionally associated with IgA. In this review, we summarize our current understanding of IgG and FcγR function at this unique host-environment interface, from the pathogenesis of colitis and defense against enteropathogens, its contribution to maternal-fetal cross-talk and susceptibility to cancer. Finally, we discuss the therapeutic implications of this information, both in terms of how FcγR signaling pathways may be targeted for the treatment of IBD and how FcγR engagement may influence the efficacy of therapeutic monoclonal antibodies in IBD.
Keywords: Fcγ receptor; IgG; inflammatory bowel disease; intestinal immunity; mucosal infections; neonatal immunity.
Figures
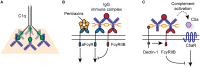
Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
How to create accessible research summaries for the developmental language disorder community.Int J Lang Commun Disord. 2025 Jan-Feb;60(1):e13142. doi: 10.1111/1460-6984.13142. Int J Lang Commun Disord. 2025. PMID: 39625399
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
Cited by
-
Investigating the shared genetic basis of inflammatory bowel disease and systemic lupus erythematosus using genetic overlap analysis.BMC Genomics. 2024 Sep 16;25(1):868. doi: 10.1186/s12864-024-10787-0. BMC Genomics. 2024. PMID: 39285290 Free PMC article.
-
Differentiation of T Helper 17 Cells May Mediate the Abnormal Humoral Immunity in IgA Nephropathy and Inflammatory Bowel Disease Based on Shared Genetic Effects.Front Immunol. 2022 Jun 13;13:916934. doi: 10.3389/fimmu.2022.916934. eCollection 2022. Front Immunol. 2022. PMID: 35769467 Free PMC article.
-
Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report.Lancet. 2023 Jul 29;402(10399):397-410. doi: 10.1016/S0140-6736(23)00775-4. Epub 2023 Jun 29. Lancet. 2023. PMID: 37393920 Free PMC article.
-
Inter-individual and inter-regional variability of breast milk antibody reactivity to bacterial lipopolysaccharides.Front Immunol. 2024 Sep 6;15:1404192. doi: 10.3389/fimmu.2024.1404192. eCollection 2024. Front Immunol. 2024. PMID: 39308863 Free PMC article.
-
ICOS ligand and IL-10 synergize to promote host-microbiota mutualism.Proc Natl Acad Sci U S A. 2021 Mar 30;118(13):e2018278118. doi: 10.1073/pnas.2018278118. Proc Natl Acad Sci U S A. 2021. PMID: 33753483 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

