Justification of specific genetic modifications in pigs for clinical organ xenotransplantation
- PMID: 30989742
- PMCID: PMC10154075
- DOI: 10.1111/xen.12516
Justification of specific genetic modifications in pigs for clinical organ xenotransplantation
Abstract
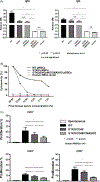
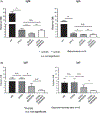
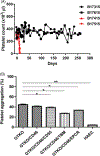
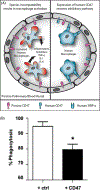
Xenotransplantation research has made considerable progress in recent years, largely through the increasing availability of pigs with multiple genetic modifications. We suggest that a pig with nine genetic modifications (ie, currently available) will provide organs (initially kidneys and hearts) that would function for a clinically valuable period of time, for example, >12 months, after transplantation into patients with end-stage organ failure. The national regulatory authorities, however, will likely require evidence, based on in vitro and/or in vivo experimental data, to justify the inclusion of each individual genetic modification in the pig. We provide data both from our own experience and that of others on the advantages of pigs in which (a) all three known carbohydrate xenoantigens have been deleted (triple-knockout pigs), (b) two human complement-regulatory proteins (CD46, CD55) and two human coagulation-regulatory proteins (thrombomodulin, endothelial cell protein C receptor) are expressed, (c) the anti-apoptotic and "anti-inflammatory" molecule, human hemeoxygenase-1 is expressed, and (d) human CD47 is expressed to suppress elements of the macrophage and T-cell responses. Although many alternative genetic modifications could be made to an organ-source pig, we suggest that the genetic manipulations we identify above will all contribute to the success of the initial clinical pig kidney or heart transplants, and that the beneficial contribution of each individual manipulation is supported by considerable experimental evidence.
Keywords: clinical; genetically engineered; heart; human; kidney; pig; transgenes; triple-knockout; xenotransplantation.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
Elena Federzoni is an employee of United Therapeutics, and Amy Dandro and David Ayares are employees of Revivicor, Inc The other authors declare no conflicts of interest.
Figures
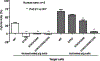

Similar articles
-
The desirable donor pig to eliminate all xenoreactive antigens.Xenotransplantation. 2019 Jul;26(4):e12504. doi: 10.1111/xen.12504. Epub 2019 Mar 2. Xenotransplantation. 2019. PMID: 30825348 Review.
-
Genetic engineering of porcine endothelial cell lines for evaluation of human-to-pig xenoreactive immune responses.Sci Rep. 2021 Jun 23;11(1):13131. doi: 10.1038/s41598-021-92543-y. Sci Rep. 2021. PMID: 34162938 Free PMC article.
-
Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation.J Thorac Cardiovasc Surg. 2014 Sep;148(3):1106-13; discussion 1113-4. doi: 10.1016/j.jtcvs.2014.06.002. Epub 2014 Jun 6. J Thorac Cardiovasc Surg. 2014. PMID: 24998698 Free PMC article.
-
Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood.Xenotransplantation. 2015 Mar-Apr;22(2):102-11. doi: 10.1111/xen.12149. Epub 2014 Dec 2. Xenotransplantation. 2015. PMID: 25470239 Free PMC article.
-
Modifying the sugar icing on the transplantation cake.Glycobiology. 2016 Jun;26(6):571-81. doi: 10.1093/glycob/cww028. Epub 2016 Mar 1. Glycobiology. 2016. PMID: 26935763 Free PMC article. Review.
Cited by
-
Aspects of histocompatibility testing in xenotransplantation.Transpl Immunol. 2021 Aug;67:101409. doi: 10.1016/j.trim.2021.101409. Epub 2021 May 17. Transpl Immunol. 2021. PMID: 34015463 Free PMC article. Review.
-
"You cannot stay in the laboratory forever"*: Taking pig kidney xenotransplantation from the laboratory to the clinic.EBioMedicine. 2021 Sep;71:103562. doi: 10.1016/j.ebiom.2021.103562. Epub 2021 Sep 10. EBioMedicine. 2021. PMID: 34517284 Free PMC article. Review.
-
The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors.J Thorac Cardiovasc Surg. 2023 Feb;165(2):e69-e81. doi: 10.1016/j.jtcvs.2021.07.051. Epub 2021 Sep 4. J Thorac Cardiovasc Surg. 2023. PMID: 34579956 Free PMC article.
-
Characterisation of transgenic pigs expressing a human T cell-depleting anti-CD2 monoclonal antibody.Xenotransplantation. 2023 Nov 13;31(1):e12836. doi: 10.1111/xen.12836. Online ahead of print. Xenotransplantation. 2023. PMID: 37961013 Free PMC article.
-
Current Topics of Relevance to the Xenotransplantation of Free Pig Islets.Front Immunol. 2022 Apr 1;13:854883. doi: 10.3389/fimmu.2022.854883. eCollection 2022. Front Immunol. 2022. PMID: 35432379 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

