A clinically relevant murine model unmasks a "two-hit" mechanism for reactivation and dissemination of cytomegalovirus after kidney transplant
- PMID: 30947382
- PMCID: PMC6873708
- DOI: 10.1111/ajt.15376
A clinically relevant murine model unmasks a "two-hit" mechanism for reactivation and dissemination of cytomegalovirus after kidney transplant
Erratum in
-
Erratum.Am J Transplant. 2020 Mar;20(3):907. doi: 10.1111/ajt.15593. Epub 2019 Sep 19. Am J Transplant. 2020. PMID: 32090475 No abstract available.
Abstract
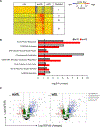
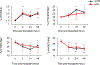
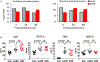
Reactivation of latent cytomegalovirus remains an important complication after transplant. Although immunosuppression (IS) has been implicated as a primary cause, we have previously shown that the implantation response of a kidney allograft can lead to early transcriptional activation of latent murine cytomegalovirus (MCMV) genes in an immune-competent host and to MCMV reactivation and dissemination to other organs in a genetically immune-deficient recipient. We now describe a model that allows us to separately analyze the impact of the implantation effect vs that of a clinically relevant IS regimen. Treatment with IS of latently infected mice alone does not induce viral reactivation, but transplant of latently infected allogeneic kidneys combined with IS facilitates MCMV reactivation in the graft and dissemination to other organs. The IS regimen effectively dampens allo-immune inflammatory pathways and depletes recipient anti-MCMV but does not affect ischemia-reperfusion injury pathways. MCMV reactivation similar to that seen in allogeneic transplants combined with also occurs after syngeneic transplants. Thus, our data strongly suggest that while ischemia-reperfusion injury of the implanted graft is sufficient and necessary to initiate transcriptional reactivation of latent MCMV ("first hit"), IS is permissive to the first hit and facilitates dissemination to other organs ("second hit").
Keywords: animal models: murine; basic (laboratory) research/science; immunosuppression/immune modulation; immunosuppressive regimens; infection and infectious agents - viral: Cytomegalovirus (CMV); infectious disease; ischemia reperfusion injury (IRI); kidney transplantation/nephrology; signaling/signaling pathways; translational research/science.
© 2019 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures



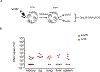

Comment in
-
Cytomegalovirus reactivation: Another reason to minimize graft ischemia/reperfusion.Am J Transplant. 2019 Sep;19(9):2399-2400. doi: 10.1111/ajt.15504. Epub 2019 Jul 18. Am J Transplant. 2019. PMID: 31215764 No abstract available.
Similar articles
-
A model for reactivation of CMV from latency.J Clin Virol. 2002 Aug;25 Suppl 2:S123-36. doi: 10.1016/s1386-6532(02)00088-4. J Clin Virol. 2002. PMID: 12361763 Review.
-
A mouse model of CMV transmission following kidney transplantation.Am J Transplant. 2012 Apr;12(4):1024-8. doi: 10.1111/j.1600-6143.2011.03892.x. Epub 2012 Jan 6. Am J Transplant. 2012. PMID: 22226173
-
Allogeneic stimulation causes transcriptional reactivation of latent murine cytomegalovirus.Transplant Proc. 2009 Jun;41(5):1927-31. doi: 10.1016/j.transproceed.2009.02.086. Transplant Proc. 2009. PMID: 19545758 Free PMC article.
-
Murine cytomegalovirus dissemination but not reactivation in donor-positive/recipient-negative allogeneic kidney transplantation can be effectively prevented by transplant immune tolerance.Kidney Int. 2020 Jul;98(1):147-158. doi: 10.1016/j.kint.2020.01.034. Epub 2020 Feb 21. Kidney Int. 2020. PMID: 32471635 Free PMC article.
-
New Insights Into the Molecular Mechanisms and Immune Control of Cytomegalovirus Reactivation.Transplantation. 2020 May;104(5):e118-e124. doi: 10.1097/TP.0000000000003138. Transplantation. 2020. PMID: 31996662 Free PMC article. Review.
Cited by
-
The Human Cytomegalovirus β2.7 Long Non-Coding RNA Prevents Induction of Reactive Oxygen Species to Maintain Viral Gene Silencing during Latency.Int J Mol Sci. 2022 Sep 20;23(19):11017. doi: 10.3390/ijms231911017. Int J Mol Sci. 2022. PMID: 36232315 Free PMC article.
-
Murine cytomegalovirus promotes renal allograft inflammation via Th1/17 cells and IL-17A.Am J Transplant. 2022 Oct;22(10):2306-2322. doi: 10.1111/ajt.17116. Epub 2022 Jun 15. Am J Transplant. 2022. PMID: 35671112 Free PMC article.
-
Cytomegalovirus in urinary sediment in patients with acute kidney injury.BMC Nephrol. 2021 May 8;22(1):169. doi: 10.1186/s12882-021-02377-z. BMC Nephrol. 2021. PMID: 33962580 Free PMC article.
-
Blocking the IL-1 receptor reduces cardiac transplant ischemia and reperfusion injury and mitigates CMV-accelerated chronic rejection.Am J Transplant. 2021 Jan;21(1):44-59. doi: 10.1111/ajt.16149. Epub 2020 Jul 18. Am J Transplant. 2021. PMID: 33405337 Free PMC article.
-
Resilience of T cell-intrinsic dysfunction in transplantation tolerance.Proc Natl Acad Sci U S A. 2019 Nov 19;116(47):23682-23690. doi: 10.1073/pnas.1910298116. Epub 2019 Nov 4. Proc Natl Acad Sci U S A. 2019. PMID: 31685610 Free PMC article.
References
-
- Mocarski E, Shenk T, Griffiths PD, Pass RF. Cytomegaloviruses. In: Knipe DM, ed. Fields Virology. Vol 2, 6 ed. Philadelphia: Wolters Kluwer Health; 2013:1960–2014.
-
- Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med 1998;338(24):1741–1751. - PubMed
-
- Razonable RR, Humar A, the AST Infectious Disease Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13(suppl 4):93–106. - PubMed
-
- Kaminski H, Fishman JA. The cell biology of cytomegalovirus: implications for transplantation. Am J Transplant. 2016;16(8):2254–2269. - PubMed

