PGC1α/CEBPB/CPT1A axis promotes radiation resistance of nasopharyngeal carcinoma through activating fatty acid oxidation
- PMID: 30945396
- PMCID: PMC6550130
- DOI: 10.1111/cas.14011
PGC1α/CEBPB/CPT1A axis promotes radiation resistance of nasopharyngeal carcinoma through activating fatty acid oxidation
Abstract
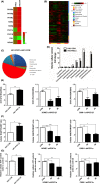
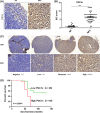
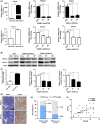
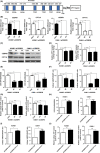
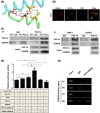
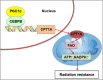
The PPAR coactivator-1α (PGC1α) is an important transcriptional co-activator in control of fatty acid metabolism. Mitochondrial fatty acid oxidation (FAO) is the primary pathway for the degradation of fatty acids and promotes NADPH and ATP production. Our previous study demonstrated that upregulation of carnitine palmitoyl transferase 1 A (CPT1A), the key regulator of FAO, promotes radiation resistance of nasopharyngeal carcinoma (NPC). In this study, we found that high expression of PGC1α is associated with poor overall survival in NPC patients after radiation treatment. Targeting PGC1α could sensitize NPC cells to radiotherapy. Mechanically, PGC1α binds to CCAAT/enhancer binding protein β (CEBPB), a member of the transcription factor family of CEBP, to promote CPT1A transcription, resulting in activation of FAO. Our results revealed that the PGC1α/CEBPB/CPT1A/FAO signaling axis promotes radiation resistance of NPC. These findings indicate that the expression of PGC1α could be a prognostic indicator of NPC, and targeting FAO in NPC with high expression of PGC1α might improve the therapeutic efficacy of radiotherapy.
Keywords: CPT1A; PGC1α; fatty acid oxidation; nasopharyngeal carcinoma; radiation therapy.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

Similar articles
-
Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy.Theranostics. 2018 Mar 22;8(9):2329-2347. doi: 10.7150/thno.21451. eCollection 2018. Theranostics. 2018. PMID: 29721083 Free PMC article.
-
PGC1α-mediated fatty acid oxidation promotes TGFβ1-induced epithelial-mesenchymal transition and metastasis of nasopharyngeal carcinoma.Life Sci. 2022 Jul 1;300:120558. doi: 10.1016/j.lfs.2022.120558. Epub 2022 Apr 19. Life Sci. 2022. PMID: 35452637
-
CPT1A-mediated fatty acid oxidation promotes cell proliferation via nucleoside metabolism in nasopharyngeal carcinoma.Cell Death Dis. 2022 Apr 11;13(4):331. doi: 10.1038/s41419-022-04730-y. Cell Death Dis. 2022. PMID: 35411000 Free PMC article.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
-
CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential.Endocrinology. 2020 Feb 1;161(2):bqz046. doi: 10.1210/endocr/bqz046. Endocrinology. 2020. PMID: 31900483 Review.
Cited by
-
Perspectives of lipid metabolism reprogramming in head and neck squamous cell carcinoma: An overview.Front Oncol. 2022 Sep 16;12:1008361. doi: 10.3389/fonc.2022.1008361. eCollection 2022. Front Oncol. 2022. PMID: 36185215 Free PMC article. Review.
-
Inducing disulfidptosis in tumors:potential pathways and significance.MedComm (2020). 2024 Oct 15;5(11):e791. doi: 10.1002/mco2.791. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39415848 Free PMC article. Review.
-
Wild-type IDH2 contributes to Epstein-Barr virus-dependent metabolic alterations and tumorigenesis.Mol Metab. 2020 Jun;36:100966. doi: 10.1016/j.molmet.2020.02.009. Epub 2020 Feb 18. Mol Metab. 2020. PMID: 32224436 Free PMC article.
-
CPT1A and fatty acid β-oxidation are essential for tumor cell growth and survival in hormone receptor-positive breast cancer.NAR Cancer. 2021 Sep 9;3(3):zcab035. doi: 10.1093/narcan/zcab035. eCollection 2021 Sep. NAR Cancer. 2021. PMID: 34514415 Free PMC article.
-
Targeting the signaling in Epstein-Barr virus-associated diseases: mechanism, regulation, and clinical study.Signal Transduct Target Ther. 2021 Jan 12;6(1):15. doi: 10.1038/s41392-020-00376-4. Signal Transduct Target Ther. 2021. PMID: 33436584 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

