Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program
- PMID: 30944023
- PMCID: PMC6448290
- DOI: 10.1186/s40425-019-0579-z
Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: the Italian expanded access program
Abstract
Background: The Italian Renal Cell Cancer Early Access Program was an expanded access program that allowed access to nivolumab, for patients (pts) with metastatic renal cell carcinoma (mRCC) prior to regulatory approval.
Methods: Pts with previously treated advanced or mRCC were eligible to receive nivolumab 3 mg/kg every 2 weeks. Pts included in the analysis had received ≥1 dose of nivolumab and were monitored for drug-related adverse events (drAEs) using CTCAE v.4.0. Immune-related (ir) AEs were defined as AEs displaying a certain, likely or possible correlation with immunotherapy (cutaneous, endocrine, hepatic, gastro-intestinal and pulmonary). The association between overall survival (OS) and irAEs was assessed, and associations between variables were evaluated with a logistic regression model.
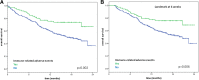
Results: A total of 389 pts were enrolled between July 2015 and April 2016. Overall, the objective response rate was 23.1%. At a median follow-up of 12 months, the median progression-free survival was 4.5 months (95% CI 3.7-6.2) and the 12-month overall survival rate was 63%. Any grade and grade 3-4 drAEs were reported in 124 (32%) and 27 (7%) of pts, respectively, and there were no treatment-related deaths. Any grade irAEs occurred in 76 (20%) of patients, 8% cutaneous, 4% endocrine, 2% hepatic, 5% gastro-intestinal and 1% pulmonary. Of the 22 drAEs inducing treatment discontinuation, 10 (45%) were irAEs. Pts with drAEs had a significantly longer survival than those without drAEs (median OS 22.5 versus 16.4 months, p = 0.01). Pts with irAEs versus without irAEs had a more significant survival benefit (median OS not reached versus 16.8 months, p = 0.002), confirmed at the landmark analysis at 6 weeks. The occurrence of irAEs displayed a strong association with OS in univariable (HR 0.48, p = 0.003) and multivariable (HR 0.57, p = 0.02) analysis.
Conclusions: The appearance of irAEs strongly correlates with survival benefit in a real-life population of mRCC pts treated with nivolumab.
Keywords: Adverse events; Expanded access trials; Immunotherapy; Renal cell carcinoma.
Conflict of interest statement
Ethics approval and consent to participate
The Early Access Program for nivolumab received the approval by the Ethics Committee of each Italian Center included in the program.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Safety and efficacy of nivolumab for metastatic renal cell carcinoma: real-world results from an expanded access programme.BJU Int. 2019 Jan;123(1):98-105. doi: 10.1111/bju.14461. Epub 2018 Jul 23. BJU Int. 2019. PMID: 29956884
-
Association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab.Urol Oncol. 2019 Jun;37(6):355.e21-355.e29. doi: 10.1016/j.urolonc.2019.03.003. Epub 2019 Mar 29. Urol Oncol. 2019. PMID: 30935847
-
Efficacy and safety data in elderly patients with metastatic renal cell carcinoma included in the nivolumab Expanded Access Program (EAP) in Italy.PLoS One. 2018 Jul 6;13(7):e0199642. doi: 10.1371/journal.pone.0199642. eCollection 2018. PLoS One. 2018. PMID: 29979712 Free PMC article.
-
Outcomes and Safety of Biweekly and Monthly Nivolumab in Patients with Metastatic Renal Cell Carcinoma and Dialysis: Three Case Reports and Literature Review.Urol Int. 2020;104(3-4):323-326. doi: 10.1159/000504515. Epub 2020 Jan 8. Urol Int. 2020. PMID: 31914452 Review.
-
Immunotherapy in Dialysis-Dependent Cancer Patients: Our Experience in Patients With Metastatic Renal Cell Carcinoma and a Review of the Literature.Clin Genitourin Cancer. 2019 Oct;17(5):e903-e908. doi: 10.1016/j.clgc.2019.06.009. Epub 2019 Jul 8. Clin Genitourin Cancer. 2019. PMID: 31375351 Review. No abstract available.
Cited by
-
Immune-Related Adverse Events Induced by Immune Checkpoint Inhibitors and CAR-T Cell Therapy: A Comprehensive Imaging-Based Review.Cancers (Basel). 2024 Jul 19;16(14):2585. doi: 10.3390/cancers16142585. Cancers (Basel). 2024. PMID: 39061225 Free PMC article. Review.
-
Checkpoint Inhibitor-Induced Colitis-A Clinical Overview of Incidence, Prognostic Implications and Extension of Current Treatment Options.Pharmaceuticals (Basel). 2021 Apr 16;14(4):367. doi: 10.3390/ph14040367. Pharmaceuticals (Basel). 2021. PMID: 33923423 Free PMC article. Review.
-
Association between Immune Related Adverse Events and Outcome in Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors.Cancers (Basel). 2021 Feb 18;13(4):860. doi: 10.3390/cancers13040860. Cancers (Basel). 2021. PMID: 33670634 Free PMC article.
-
Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors.J Immunother Cancer. 2019 Nov 15;7(1):306. doi: 10.1186/s40425-019-0805-8. J Immunother Cancer. 2019. PMID: 31730012 Free PMC article. Review.
-
Association between Immune-Related Adverse Events and Survival in 319 Stage IV Melanoma Patients Treated with PD-1-Based Immunotherapy: An Approach Based on Clinical Chemistry.Cancers (Basel). 2021 Dec 6;13(23):6141. doi: 10.3390/cancers13236141. Cancers (Basel). 2021. PMID: 34885249 Free PMC article.
References
-
- Escudier B, Sharma P, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for Nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol. 2017;72(6):962–971. doi: 10.1016/j.eururo.2017.02.010. - DOI - PubMed
-
- Administration USFD, FDA Expands Use of Immunotherapeutic to kidney cancer. http://blog.aacr.org/fda-approval-nivolumab-kidney-cancer/ 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous