Neddylation: a novel modulator of the tumor microenvironment
- PMID: 30943988
- PMCID: PMC6446326
- DOI: 10.1186/s12943-019-0979-1
Neddylation: a novel modulator of the tumor microenvironment
Abstract
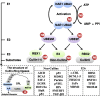
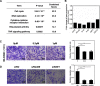
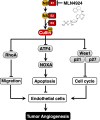
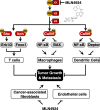
Neddylation, a post-translational modification that adds an ubiquitin-like protein NEDD8 to substrate proteins, modulates many important biological processes, including tumorigenesis. The process of protein neddylation is overactivated in multiple human cancers, providing a sound rationale for its targeting as an attractive anticancer therapeutic strategy, as evidence by the development of NEDD8-activating enzyme (NAE) inhibitor MLN4924 (also known as pevonedistat). Neddylation inhibition by MLN4924 exerts significantly anticancer effects mainly by triggering cell apoptosis, senescence and autophagy. Recently, intensive evidences reveal that inhibition of neddylation pathway, in addition to acting on tumor cells, also influences the functions of multiple important components of the tumor microenvironment (TME), including immune cells, cancer-associated fibroblasts (CAFs), cancer-associated endothelial cells (CAEs) and some factors, all of which are crucial for tumorigenesis. Here, we briefly summarize the latest progresses in this field to clarify the roles of neddylation in the TME, thus highlighting the overall anticancer efficacy of neddylaton inhibition.
Keywords: Cancer-associated endothelial cells; Cancer-associated fibroblasts; Immune cells; Neddylation; Tumor microenvironment; Tumor-derived factors.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Targeting Protein Neddylation for Cancer Therapy.Adv Exp Med Biol. 2020;1217:297-315. doi: 10.1007/978-981-15-1025-0_18. Adv Exp Med Biol. 2020. PMID: 31898235 Review.
-
Protein neddylation and its alterations in human cancers for targeted therapy.Cell Signal. 2018 Apr;44:92-102. doi: 10.1016/j.cellsig.2018.01.009. Epub 2018 Jan 10. Cell Signal. 2018. PMID: 29331584 Free PMC article. Review.
-
Neddylation Pathway as a Novel Anti-cancer Target: Mechanistic Investigation and Therapeutic Implication.Anticancer Agents Med Chem. 2015;15(9):1127-33. doi: 10.2174/1871520615666150305111257. Anticancer Agents Med Chem. 2015. PMID: 25742093 Review.
-
Validation of NEDD8-conjugating enzyme UBC12 as a new therapeutic target in lung cancer.EBioMedicine. 2019 Jul;45:81-91. doi: 10.1016/j.ebiom.2019.06.005. Epub 2019 Jun 14. EBioMedicine. 2019. PMID: 31208947 Free PMC article.
-
The Double-Edged Effects of MLN4924: Rethinking Anti-Cancer Drugs Targeting the Neddylation Pathway.Biomolecules. 2024 Jun 21;14(7):738. doi: 10.3390/biom14070738. Biomolecules. 2024. PMID: 39062453 Free PMC article. Review.
Cited by
-
Function, mechanism and drug discovery of ubiquitin and ubiquitin-like modification with multiomics profiling for cancer therapy.Acta Pharm Sin B. 2023 Nov;13(11):4341-4372. doi: 10.1016/j.apsb.2023.07.019. Epub 2023 Jul 22. Acta Pharm Sin B. 2023. PMID: 37969742 Free PMC article. Review.
-
UBE2M forms a positive feedback loop with estrogen receptor to drive breast cancer progression and drug resistance.Cell Death Dis. 2024 Aug 13;15(8):590. doi: 10.1038/s41419-024-06979-x. Cell Death Dis. 2024. PMID: 39138151 Free PMC article.
-
Ribosome heterogeneity in development and disease.Front Cell Dev Biol. 2024 Jul 17;12:1414269. doi: 10.3389/fcell.2024.1414269. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39086661 Free PMC article.
-
NEDD8-conjugating enzyme E2s: critical targets for cancer therapy.Cell Death Discov. 2023 Jan 23;9(1):23. doi: 10.1038/s41420-023-01337-w. Cell Death Discov. 2023. PMID: 36690633 Free PMC article. Review.
-
Abnormal Cullin1 neddylation-mediated p21 accumulation participates in the pathogenesis of recurrent spontaneous abortion by regulating trophoblast cell proliferation and differentiation.Mol Hum Reprod. 2020 May 15;26(5):327-339. doi: 10.1093/molehr/gaaa021. Mol Hum Reprod. 2020. PMID: 32186736 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

