STIM-Orai Channels and Reactive Oxygen Species in the Tumor Microenvironment
- PMID: 30935064
- PMCID: PMC6520831
- DOI: 10.3390/cancers11040457
STIM-Orai Channels and Reactive Oxygen Species in the Tumor Microenvironment
Abstract
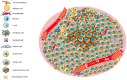
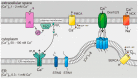
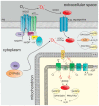
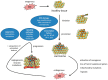
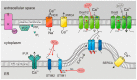
The tumor microenvironment (TME) is shaped by cancer and noncancerous cells, the extracellular matrix, soluble factors, and blood vessels. Interactions between the cells, matrix, soluble factors, and blood vessels generate this complex heterogeneous microenvironment. The TME may be metabolically beneficial or unbeneficial for tumor growth, it may favor or not favor a productive immune response against tumor cells, or it may even favor conditions suited to hijacking the immune system for benefitting tumor growth. Soluble factors relevant for TME include oxygen, reactive oxygen species (ROS), ATP, Ca2+, H⁺, growth factors, or cytokines. Ca2+ plays a prominent role in the TME because its concentration is directly linked to cancer cell proliferation, apoptosis, or migration but also to immune cell function. Stromal-interaction molecules (STIM)-activated Orai channels are major Ca2+ entry channels in cancer cells and immune cells, they are upregulated in many tumors, and they are strongly regulated by ROS. Thus, STIM and Orai are interesting candidates to regulate cancer cell fate in the TME. In this review, we summarize the current knowledge about the function of ROS and STIM/Orai in cancer cells; discuss their interdependencies; and propose new hypotheses how TME, ROS, and Orai channels influence each other.
Keywords: H2O2; Orai; STIM; calcium; reactive oxygen species; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of Orai/STIM Channels by pH.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 9. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 9. PMID: 30299649 Free Books & Documents. Review.
-
Studies of Structure-Function and Subunit Composition of Orai/STIM Channel.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 2. PMID: 30299645 Free Books & Documents. Review.
-
Store-Independent Orai Channels Regulated by STIM.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
-
Redox modulation of STIM-ORAI signaling.Cell Calcium. 2016 Aug;60(2):142-52. doi: 10.1016/j.ceca.2016.03.006. Epub 2016 Mar 19. Cell Calcium. 2016. PMID: 27041216 Review.
-
The role of Orai-STIM calcium channels in melanocytes and melanoma.J Physiol. 2016 Jun 1;594(11):2825-35. doi: 10.1113/JP271141. Epub 2016 Apr 6. J Physiol. 2016. PMID: 26864956 Free PMC article. Review.
Cited by
-
Red blood cell-derived materials for cancer therapy: Construction, distribution, and applications.Mater Today Bio. 2023 Dec 15;24:100913. doi: 10.1016/j.mtbio.2023.100913. eCollection 2024 Feb. Mater Today Bio. 2023. PMID: 38188647 Free PMC article. Review.
-
Bipolar disorder-iPSC derived neural progenitor cells exhibit dysregulation of store-operated Ca2+ entry and accelerated differentiation.Mol Psychiatry. 2023 Dec;28(12):5237-5250. doi: 10.1038/s41380-023-02152-6. Epub 2023 Jul 4. Mol Psychiatry. 2023. PMID: 37402854 Free PMC article.
-
Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics.Sensors (Basel). 2024 Apr 30;24(9):2865. doi: 10.3390/s24092865. Sensors (Basel). 2024. PMID: 38732975 Free PMC article. Review.
-
Orai, STIM, and PMCA contribute to reduced calcium signal generation in CD8+ T cells of elderly mice.Aging (Albany NY). 2020 Feb 12;12(4):3266-3286. doi: 10.18632/aging.102809. Epub 2020 Feb 12. Aging (Albany NY). 2020. PMID: 32062611 Free PMC article.
-
Hyaluronic Acid Improves Hydrogen Peroxide Modulatory Effects on Calcium Channel and Sodium-Potassium Pump in 4T1 Breast Cancer Cell Line.Oxid Med Cell Longev. 2020 Dec 30;2020:8681349. doi: 10.1155/2020/8681349. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33456676 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

