Leishmania donovani 90 kD Heat Shock Protein - Impact of Phosphosites on Parasite Fitness, Infectivity and Casein Kinase Affinity
- PMID: 30911045
- PMCID: PMC6434042
- DOI: 10.1038/s41598-019-41640-0
Leishmania donovani 90 kD Heat Shock Protein - Impact of Phosphosites on Parasite Fitness, Infectivity and Casein Kinase Affinity
Abstract
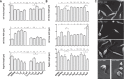
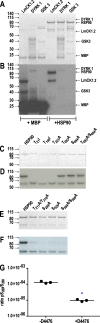
Leishmania parasites are thought to control protein activity at the post-translational level, e.g. by protein phosphorylation. In the pathogenic amastigote, the mammalian stage of Leishmania parasites, heat shock proteins show increased phosphorylation, indicating a role in stage-specific signal transduction. Here we investigate the impact of phosphosites in the L. donovani heat shock protein 90. Using a chemical knock-down/genetic complementation approach, we mutated 11 confirmed or presumed phosphorylation sites and assessed the impact on overall fitness, morphology and in vitro infectivity. Most phosphosite mutations affected the growth and morphology of promastigotes in vitro, but with one exception, none of the phosphorylation site mutants had a selective impact on the in vitro infection of macrophages. Surprisingly, aspartate replacements mimicking the negative charge of phosphorylated serines or threonines had mostly negative impacts on viability and infectivity. HSP90 is a substrate for casein kinase 1.2-catalysed phosphorylation in vitro. While several putative phosphosite mutations abrogated casein kinase 1.2 activity on HSP90, only Ser289 could be identified as casein kinase target by mass spectrometry. In summary, our data show HSP90 as a downstream client of phosphorylation-mediated signalling in an organism that depends on post-transcriptional gene regulation.
Conflict of interest statement
The authors declare no competing interests.
Figures

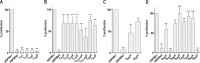

Similar articles
-
Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani.Mol Biol Cell. 2001 Nov;12(11):3307-16. doi: 10.1091/mbc.12.11.3307. Mol Biol Cell. 2001. PMID: 11694568 Free PMC article.
-
Heat-stress induced modulation of protein phosphorylation in virulent promastigotes of Leishmania donovani.Int J Biochem Cell Biol. 2000 Mar;32(3):309-16. doi: 10.1016/s1357-2725(99)00134-x. Int J Biochem Cell Biol. 2000. PMID: 10716628
-
Leishmania donovani P23 protects parasites against HSP90 inhibitor-mediated growth arrest.Cell Stress Chaperones. 2015 Jul;20(4):673-85. doi: 10.1007/s12192-015-0595-y. Epub 2015 May 7. Cell Stress Chaperones. 2015. PMID: 25948161 Free PMC article.
-
MAPK1 of Leishmania donovani interacts and phosphorylates HSP70 and HSP90 subunits of foldosome complex.Sci Rep. 2017 Aug 31;7(1):10202. doi: 10.1038/s41598-017-09725-w. Sci Rep. 2017. PMID: 28860596 Free PMC article.
-
Antiparasitic chemotherapy: tinkering with the purine salvage pathway.Adv Exp Med Biol. 2008;625:116-32. doi: 10.1007/978-0-387-77570-8_10. Adv Exp Med Biol. 2008. PMID: 18365663 Review.
Cited by
-
Absence of DEATH kinesin is fatal for Leishmania mexicana amastigotes.Sci Rep. 2022 Feb 28;12(1):3266. doi: 10.1038/s41598-022-07412-z. Sci Rep. 2022. PMID: 35228627 Free PMC article.
-
Genomic Insight of Leishmania Parasite: In-Depth Review of Drug Resistance Mechanisms and Genetic Mutations.ACS Omega. 2024 Mar 8;9(11):12500-12514. doi: 10.1021/acsomega.3c09400. eCollection 2024 Mar 19. ACS Omega. 2024. PMID: 38524425 Free PMC article. Review.
-
Heat Shock Proteins as the Druggable Targets in Leishmaniasis: Promises and Perils.Infect Immun. 2021 Jan 19;89(2):e00559-20. doi: 10.1128/IAI.00559-20. Print 2021 Jan 19. Infect Immun. 2021. PMID: 33139381 Free PMC article. Review.
-
Structure, regulation, and (patho-)physiological functions of the stress-induced protein kinase CK1 delta (CSNK1D).Gene. 2019 Oct 5;715:144005. doi: 10.1016/j.gene.2019.144005. Epub 2019 Jul 31. Gene. 2019. PMID: 31376410 Free PMC article. Review.
-
Leishmania Protein Kinases: Important Regulators of the Parasite Life Cycle and Molecular Targets for Treating Leishmaniasis.Microorganisms. 2021 Mar 27;9(4):691. doi: 10.3390/microorganisms9040691. Microorganisms. 2021. PMID: 33801655 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

