SUMOylation promotes survival and integration of neural stem cell grafts in ischemic stroke
- PMID: 30905846
- PMCID: PMC6491415
- DOI: 10.1016/j.ebiom.2019.03.035
SUMOylation promotes survival and integration of neural stem cell grafts in ischemic stroke
Abstract
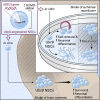
Background: Neural stem cell (NSC)-based therapies hold great promise for treating diseases of the central nervous system (CNS). However, several fundamental problems still need to be overcome to fully exploit the clinical potential of NSC therapeutics. Chief among them is the limited survival of NSC grafts within hostile microenvironments.
Methods: Herein, we sought to engineer NSCs in an effort to increase graft survival within ischemic brain lesions via upregulation of global SUMOylation, a post-translational modification critically involved in mediating tolerance to ischemia/reperfusion.
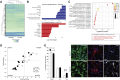
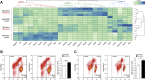
Findings: NSCs overexpressing the SUMO E2-conjugase Ubc9 displayed resistance to oxygen-glucose-deprivation/restoration of oxygen/glucose (OGD/ROG) and enhanced neuronal differentiation in vitro, as well as increased survival and neuronal differentiation when transplanted in mice with transient middle cerebral artery occlusion in vivo.
Interpretation: Our work highlights a critical role for SUMOylation in NSC biology and identifies a biological pathway that can be targeted to increase the effectiveness of exogenous stem cell medicines in ischemic stroke. FUND: Intramural Research Program of the NINDS/NIH, the Italian Multiple Sclerosis Foundation (FISM), the Bascule Charitable Trust, NIH-IRTA-OxCam and Wellcome Trust Research Training Fellowships.
Keywords: Cell therapy; Cellular engineering; Ischemia/reperfusion; Neural stem cells (NSCs); Regenerative medicine; SUMOylation; Stroke; Ubc9.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Mild Hypothermia Promotes Ischemic Tolerance and Survival of Neural Stem Cell Grafts by Enhancing Global SUMOylation.Oxid Med Cell Longev. 2022 May 27;2022:6503504. doi: 10.1155/2022/6503504. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2023 Dec 29;2023:9894206. doi: 10.1155/2023/9894206 PMID: 35669854 Free PMC article. Retracted.
-
SUMOylation of Warts kinase promotes neural stem cell reactivation.Nat Commun. 2024 Oct 17;15(1):8557. doi: 10.1038/s41467-024-52569-y. Nat Commun. 2024. PMID: 39419973 Free PMC article.
-
Neural stem cells genetically modified to overexpress cu/zn-superoxide dismutase enhance amelioration of ischemic stroke in mice.Stroke. 2012 Sep;43(9):2423-9. doi: 10.1161/STROKEAHA.112.656900. Epub 2012 Jun 19. Stroke. 2012. PMID: 22713489 Free PMC article.
-
SUMO and ischemic tolerance.Neuromolecular Med. 2013 Dec;15(4):771-81. doi: 10.1007/s12017-013-8239-9. Epub 2013 Jun 18. Neuromolecular Med. 2013. PMID: 23775726 Review.
-
SUMOylation effects on neural stem cells self-renewal, differentiation, and survival.Neurosci Res. 2024 Feb;199:1-11. doi: 10.1016/j.neures.2023.09.006. Epub 2023 Sep 22. Neurosci Res. 2024. PMID: 37742800 Review.
Cited by
-
Proper progression of neurogenesis relies on a defined pattern of SUMO-modified proteins.Neural Regen Res. 2022 Aug;17(8):1731-1732. doi: 10.4103/1673-5374.332134. Neural Regen Res. 2022. PMID: 35017422 Free PMC article. No abstract available.
-
SUMOylation Modulates Reactive Oxygen Species (ROS) Levels and Acts as a Protective Mechanism in the Type 2 Model of Diabetic Peripheral Neuropathy.Cells. 2023 Oct 24;12(21):2511. doi: 10.3390/cells12212511. Cells. 2023. PMID: 37947589 Free PMC article.
-
Protein sumoylation in normal and cancer stem cells.Front Mol Biosci. 2022 Dec 19;9:1095142. doi: 10.3389/fmolb.2022.1095142. eCollection 2022. Front Mol Biosci. 2022. PMID: 36601585 Free PMC article. Review.
-
Insight Into the Mechanisms and the Challenges on Stem Cell-Based Therapies for Cerebral Ischemic Stroke.Front Cell Neurosci. 2021 Feb 25;15:637210. doi: 10.3389/fncel.2021.637210. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33732111 Free PMC article.
-
Neural Stem Cells Therapy for Ischemic Stroke: Progress and Challenges.Transl Stroke Res. 2022 Oct;13(5):665-675. doi: 10.1007/s12975-022-00984-y. Epub 2022 Jan 15. Transl Stroke Res. 2022. PMID: 35032307 Review.
References
-
- Bacigaluppi M., Pluchino S., Jametti L.P., Kilic E., Kilic U., Salani G. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132(Pt 8):2239–2251. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

