Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments
- PMID: 30902991
- PMCID: PMC6430813
- DOI: 10.1038/s41467-019-09256-0
Aedes mosquitoes acquire and transmit Zika virus by breeding in contaminated aquatic environments
Abstract
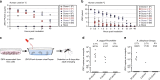
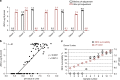
Zika virus (ZIKV) is a mosquito-borne flavivirus that predominantly circulates between humans and Aedes mosquitoes. Clinical studies have shown that Zika viruria in patients persists for an extended period, and results in infectious virions being excreted. Here, we demonstrate that Aedes mosquitoes are permissive to ZIKV infection when breeding in urine or sewage containing low concentrations of ZIKV. Mosquito larvae and pupae, including from field Aedes aegypti can acquire ZIKV from contaminated aquatic systems, resulting in ZIKV infection of adult females. Adult mosquitoes can transmit infectious virions to susceptible type I/II interferon receptor-deficient (ifnagr-/-) C57BL/6 (AG6) mice. Furthermore, ZIKV viruria from infected AG6 mice can causes mosquito infection during the aquatic life stages. Our studies suggest that infectious urine could be a natural ZIKV source, which is potentially transmissible to mosquitoes when breeding in an aquatic environment.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Microbial Composition in Larval Water Enhances Aedes aegypti Development but Reduces Transmissibility of Zika Virus.mSphere. 2021 Dec 22;6(6):e0068721. doi: 10.1128/msphere.00687-21. Epub 2021 Dec 8. mSphere. 2021. PMID: 34878293 Free PMC article.
-
Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes.Nature. 2017 May 25;545(7655):482-486. doi: 10.1038/nature22365. Epub 2017 May 17. Nature. 2017. PMID: 28514450 Free PMC article.
-
Susceptibility of Armigeres subalbatus Coquillett (Diptera: Culicidae) to Zika virus through oral and urine infection.PLoS Negl Trop Dis. 2020 Jul 6;14(7):e0008450. doi: 10.1371/journal.pntd.0008450. eCollection 2020 Jul. PLoS Negl Trop Dis. 2020. PMID: 32628662 Free PMC article.
-
Zika virus: An updated review of competent or naturally infected mosquitoes.PLoS Negl Trop Dis. 2017 Nov 16;11(11):e0005933. doi: 10.1371/journal.pntd.0005933. eCollection 2017 Nov. PLoS Negl Trop Dis. 2017. PMID: 29145400 Free PMC article. Review.
-
Zika Virus Mosquito Vectors: Competence, Biology, and Vector Control.J Infect Dis. 2017 Dec 16;216(suppl_10):S976-S990. doi: 10.1093/infdis/jix405. J Infect Dis. 2017. PMID: 29267910 Free PMC article. Review.
Cited by
-
Zika virus tropism and pathogenesis: understanding clinical impacts and transmission dynamics.Virol J. 2024 Oct 29;21(1):271. doi: 10.1186/s12985-024-02547-z. Virol J. 2024. PMID: 39472938 Free PMC article. Review.
-
West Nile virus can be transmitted within mosquito populations through infectious mosquito excreta.iScience. 2024 Oct 4;27(11):111099. doi: 10.1016/j.isci.2024.111099. eCollection 2024 Nov 15. iScience. 2024. PMID: 39473977 Free PMC article.
-
Multi-omics analysis of antiviral interactions of Elizabethkingia anophelis and Zika virus.Sci Rep. 2024 Aug 9;14(1):18470. doi: 10.1038/s41598-024-68898-3. Sci Rep. 2024. PMID: 39122799 Free PMC article.
-
Glucose-mediated proliferation of a gut commensal bacterium promotes Plasmodium infection by increasing mosquito midgut pH.Cell Rep. 2021 Apr 20;35(3):108992. doi: 10.1016/j.celrep.2021.108992. Cell Rep. 2021. PMID: 33882310 Free PMC article.
-
Armigeres subalbatus is a potential vector for Zika virus but not dengue virus.Infect Dis Poverty. 2022 Jun 4;11(1):62. doi: 10.1186/s40249-022-00990-0. Infect Dis Poverty. 2022. PMID: 35658950 Free PMC article.
References
-
- WHO. Zika Cumulative Cases, 2017. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=.... Accessed 21 Dec 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

