Targeting off-target effects: endoplasmic reticulum stress and autophagy as effective strategies to enhance temozolomide treatment
- PMID: 30881038
- PMCID: PMC6413742
- DOI: 10.2147/OTT.S194770
Targeting off-target effects: endoplasmic reticulum stress and autophagy as effective strategies to enhance temozolomide treatment
Abstract
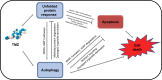
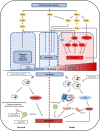
Glioblastoma multiforme (GBM) is the most common and aggressive adult primary central nervous system tumor. Unfortunately, GBM is resistant to the classic chemotherapy drug, temozolomide (TMZ). As well as its classic DNA-targeting effects, the off-target effects of TMZ can have pro-survival or pro-death roles and regulate GBM chemoradiation sensitivity. Endoplasmic reticulum (ER) stress is one of the most common off-target effects. ER stress and its downstream induction of autophagy, apoptosis, and other events have important roles in regulating TMZ sensitivity. Autophagy is an evolutionarily conserved cellular homeostasis mechanism that is closely associated with ER stress-induced apoptosis. Under ER stress, autophagy cannot only remove misfolded/unfolded proteins and damaged organelles and degrade and inhibit apoptosis-related caspase activation to reduce cell damage, but may also promote apoptosis dependent on ER stress intensity. Although some protein interactions between autophagy and apoptosis and common upstream signaling pathways have been found, the underlying regulatory mechanisms are still not fully understood. This review summarizes the possible mechanisms underlying the current known off-target roles of ER stress and downstream autophagy in the regulation of cell fate and evaluates their role in TMZ treatment and their potential as therapeutic targets.
Keywords: apoptosis; autophagy; chemotherapy resistance; endoplasmic reticulum stress; glioma; temozolomide.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Inhibition of mitochondria- and endoplasmic reticulum stress-mediated autophagy augments temozolomide-induced apoptosis in glioma cells.PLoS One. 2012;7(6):e38706. doi: 10.1371/journal.pone.0038706. Epub 2012 Jun 22. PLoS One. 2012. PMID: 22745676 Free PMC article.
-
Inhibition of phosphatidylinositol 3-kinase by PX-866 suppresses temozolomide-induced autophagy and promotes apoptosis in glioblastoma cells.Mol Med. 2019 Nov 14;25(1):49. doi: 10.1186/s10020-019-0116-z. Mol Med. 2019. PMID: 31726966 Free PMC article.
-
Chloroquine enhances temozolomide cytotoxicity in malignant gliomas by blocking autophagy.Neurosurg Focus. 2014 Dec;37(6):E12. doi: 10.3171/2014.9.FOCUS14504. Neurosurg Focus. 2014. PMID: 25434381
-
Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress.J Cell Physiol. 2017 Nov;232(11):2977-2984. doi: 10.1002/jcp.25785. Epub 2017 Mar 31. J Cell Physiol. 2017. PMID: 28067409 Review.
-
Endoplasmic reticulum stress‑induced cell death as a potential mechanism for targeted therapy in glioblastoma (Review).Int J Oncol. 2021 Aug;59(2):60. doi: 10.3892/ijo.2021.5240. Epub 2021 Jul 19. Int J Oncol. 2021. PMID: 34278449 Review.
Cited by
-
Endoplasmic Reticulum Stress Contributes to Indomethacin-Induced Glioma Apoptosis.Int J Mol Sci. 2020 Jan 15;21(2):557. doi: 10.3390/ijms21020557. Int J Mol Sci. 2020. PMID: 31952288 Free PMC article.
-
Flavopiridol Suppresses Cell Proliferation and Migration and Induces Apoptotic Cell Death by Inhibiting Oncogenic FOXM1 Signaling in IDH Wild-Type and IDH-Mutant GBM Cells.Mol Neurobiol. 2024 Feb;61(2):1061-1079. doi: 10.1007/s12035-023-03609-z. Epub 2023 Sep 7. Mol Neurobiol. 2024. PMID: 37676393
-
Targeting XBP1 mRNA splicing sensitizes glioblastoma to chemotherapy.FASEB Bioadv. 2023 Mar 13;5(5):211-220. doi: 10.1096/fba.2022-00141. eCollection 2023 May. FASEB Bioadv. 2023. PMID: 37151848 Free PMC article.
-
Inflammasome Sensor NLRP1 Confers Acquired Drug Resistance to Temozolomide in Human Melanoma.Cancers (Basel). 2020 Sep 4;12(9):2518. doi: 10.3390/cancers12092518. Cancers (Basel). 2020. PMID: 32899791 Free PMC article.
-
Four specific biomarkers associated with the progression of glioblastoma multiforme in older adults identified using weighted gene co-expression network analysis.Bioengineered. 2021 Dec;12(1):6643-6654. doi: 10.1080/21655979.2021.1975980. Bioengineered. 2021. PMID: 34516348 Free PMC article.
References
-
- Fulda S, Kögel D. Cell death by autophagy: emerging molecular mechanisms and implications for cancer therapy. Oncogene. 2015;34(40):5105–5113. - PubMed
-
- Li S, Jiang T, Li G, Wang Z. Impact of p53 status to response of temozolomide in low MGMT expression glioblastomas: preliminary results. Neurol Res. 2008;30(6):567–570. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

