The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability
- PMID: 30875900
- PMCID: PMC6468625
- DOI: 10.3390/cells8030246
The Emerging Roles of TERRA in Telomere Maintenance and Genome Stability
Abstract
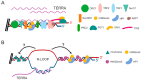
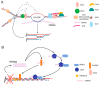
The finding that transcription occurs at chromosome ends has opened new fields of study on the roles of telomeric transcripts in chromosome end maintenance and genome stability. Indeed, the ends of chromosomes are required to be protected from activation of DNA damage response and DNA repair pathways. Chromosome end protection is achieved by the activity of specific proteins that associate with chromosome ends, forming telomeres. Telomeres need to be constantly maintained as they are in a heterochromatic state and fold into specific structures (T-loops), which may hamper DNA replication. In addition, in the absence of maintenance mechanisms, chromosome ends shorten at every cell division due to limitations in the DNA replication machinery, which is unable to fully replicate the extremities of chromosomes. Altered telomere structure or critically short chromosome ends generate dysfunctional telomeres, ultimately leading to replicative senescence or chromosome instability. Telomere biology is thus implicated in multiple human diseases, including cancer. Emerging evidence indicates that a class of long noncoding RNAs transcribed at telomeres, known as TERRA for "TElomeric Repeat-containing RNA," actively participates in the mechanisms regulating telomere maintenance and chromosome end protection. However, the molecular details of TERRA activities remain to be elucidated. In this review, we discuss recent findings on the emerging roles of TERRA in telomere maintenance and genome stability and their implications in human diseases.
Keywords: DNA damage response; TERRA; chromatin; genome stability; human diseases; long noncoding RNA; telomeres.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Telomeric repeat-containing RNA TERRA: a noncoding RNA connecting telomere biology to genome integrity.Front Genet. 2015 Apr 14;6:143. doi: 10.3389/fgene.2015.00143. eCollection 2015. Front Genet. 2015. PMID: 25926849 Free PMC article. Review.
-
Telomere transcription in ageing.Ageing Res Rev. 2020 Sep;62:101115. doi: 10.1016/j.arr.2020.101115. Epub 2020 Jun 18. Ageing Res Rev. 2020. PMID: 32565330 Review.
-
Telomeric noncoding RNA: telomeric repeat-containing RNA in telomere biology.Wiley Interdiscip Rev RNA. 2014 May-Jun;5(3):407-19. doi: 10.1002/wrna.1220. Epub 2014 Feb 12. Wiley Interdiscip Rev RNA. 2014. PMID: 24523222 Review.
-
Telomere-specific regulation of TERRA and its impact on telomere stability.Semin Cell Dev Biol. 2024 Apr;157:3-23. doi: 10.1016/j.semcdb.2023.11.001. Epub 2023 Dec 11. Semin Cell Dev Biol. 2024. PMID: 38088000 Review.
-
The makings of TERRA R-loops at chromosome ends.Cell Cycle. 2021 Sep;20(18):1745-1759. doi: 10.1080/15384101.2021.1962638. Epub 2021 Aug 25. Cell Cycle. 2021. PMID: 34432566 Free PMC article. Review.
Cited by
-
Unwrap RAP1's Mystery at Kinetoplastid Telomeres.Biomolecules. 2024 Jan 4;14(1):67. doi: 10.3390/biom14010067. Biomolecules. 2024. PMID: 38254667 Free PMC article. Review.
-
Telomere Fragility and MiDAS: Managing the Gaps at the End of the Road.Genes (Basel). 2023 Jan 29;14(2):348. doi: 10.3390/genes14020348. Genes (Basel). 2023. PMID: 36833275 Free PMC article. Review.
-
Telomere and Subtelomere R-loops and Antigenic Variation in Trypanosomes.J Mol Biol. 2020 Jul 10;432(15):4167-4185. doi: 10.1016/j.jmb.2019.10.025. Epub 2019 Nov 2. J Mol Biol. 2020. PMID: 31682833 Free PMC article. Review.
-
Telomeric-Like Repeats Flanked by Sequences Retrotranscribed from the Telomerase RNA Inserted at DNA Double-Strand Break Sites during Vertebrate Genome Evolution.Int J Mol Sci. 2021 Oct 13;22(20):11048. doi: 10.3390/ijms222011048. Int J Mol Sci. 2021. PMID: 34681704 Free PMC article.
-
Temperature-independent telomere lengthening with age in the long-lived human fish (Proteus anguinus).Proc Biol Sci. 2023 May 10;290(1998):20230503. doi: 10.1098/rspb.2023.0503. Epub 2023 May 3. Proc Biol Sci. 2023. PMID: 37132239 Free PMC article.
References
-
- Counter C.M., Avilion A.A., LeFeuvre C.E., Stewart N.G., Greider C.W., Harley C.B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

