A Novel Optical Tissue Clearing Protocol for Mouse Skeletal Muscle to Visualize Endplates in Their Tissue Context
- PMID: 30873005
- PMCID: PMC6401545
- DOI: 10.3389/fncel.2019.00049
A Novel Optical Tissue Clearing Protocol for Mouse Skeletal Muscle to Visualize Endplates in Their Tissue Context
Abstract
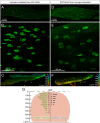
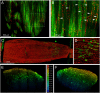
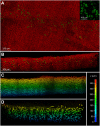
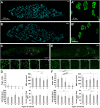
Neuromuscular junctions (NMJs) mediate skeletal muscle contractions and play an important role in several neuromuscular disorders when their morphology and function are compromised. However, due to their small size and sparse distribution throughout the comparatively large, inherently opaque muscle tissue the analysis of NMJ morphology has been limited to teased fiber preparations, longitudinal muscle sections, and flat muscles. Consequently, whole mount analyses of NMJ morphology, numbers, their distribution, and assignment to a given muscle fiber have also been impossible to determine in muscle types that are frequently used in experimental paradigms. This impossibility is exacerbated by the lack of optical tissue clearing techniques that are compatible with clear and persistent NMJ stains. Here, we present MYOCLEAR, a novel and highly reproducible muscle tissue clearing protocol. Based on hydrogel-based tissue clearing methods, this protocol permits the labeling and detection of all NMJs in adult hindleg extensor digitorum longus muscles from wildtype and diseased mice. The method is also applicable to adult mouse diaphragm muscles and can be used for different staining agents, including toxins, lectins, antibodies, and nuclear dyes. It will be useful in understanding the distribution, morphological features, and muscle tissue context of NMJs in hindleg muscle whole mounts for biomedical and basic research.
Keywords: NMJ; acetylcholine receptor; hydrogel embedding; optical tissue clearing; skeletal muscle.
Figures

Similar articles
-
Whole-mount staining of neuromuscular junctions in adult mouse diaphragms with a sandwich-like apparatus.J Neurosci Methods. 2021 Feb 15;350:109016. doi: 10.1016/j.jneumeth.2020.109016. Epub 2020 Dec 11. J Neurosci Methods. 2021. PMID: 33316317 Review.
-
Dissection of Single Skeletal Muscle Fibers for Immunofluorescent and Morphometric Analyses of Whole-Mount Neuromuscular Junctions.J Vis Exp. 2021 Aug 14;(174). doi: 10.3791/62620. J Vis Exp. 2021. PMID: 34459813
-
Postnatal Development and Distribution of Sympathetic Innervation in Mouse Skeletal Muscle.Int J Mol Sci. 2018 Jul 1;19(7):1935. doi: 10.3390/ijms19071935. Int J Mol Sci. 2018. PMID: 29966393 Free PMC article.
-
Opposite Synaptic Alterations at the Neuromuscular Junction in an ALS Mouse Model: When Motor Units Matter.J Neurosci. 2017 Sep 13;37(37):8901-8918. doi: 10.1523/JNEUROSCI.3090-16.2017. Epub 2017 Aug 11. J Neurosci. 2017. PMID: 28821658 Free PMC article.
-
What controls the position, number, size, and distribution of neuromuscular junctions on rat muscle fibers?J Neurocytol. 2003 Jun-Sep;32(5-8):835-48. doi: 10.1023/B:NEUR.0000020627.18156.b1. J Neurocytol. 2003. PMID: 15034271 Review.
Cited by
-
Routine Optical Clearing of 3D-Cell Cultures: Simplicity Forward.Front Mol Biosci. 2020 Feb 21;7:20. doi: 10.3389/fmolb.2020.00020. eCollection 2020. Front Mol Biosci. 2020. PMID: 32154265 Free PMC article.
-
Biomedical Applications of Tissue Clearing and Three-Dimensional Imaging in Health and Disease.iScience. 2020 Aug 21;23(8):101432. doi: 10.1016/j.isci.2020.101432. Epub 2020 Aug 2. iScience. 2020. PMID: 32805648 Free PMC article. Review.
-
Optical tissue clearing associated with 3D imaging: application in preclinical and clinical studies.Histochem Cell Biol. 2022 May;157(5):497-511. doi: 10.1007/s00418-022-02081-5. Epub 2022 Mar 2. Histochem Cell Biol. 2022. PMID: 35235045 Free PMC article. Review.
-
Tissue optical clearing for neural regeneration research.Neural Regen Res. 2023 Sep;18(9):1940-1941. doi: 10.4103/1673-5374.363827. Neural Regen Res. 2023. PMID: 36926711 Free PMC article. No abstract available.
-
3D Melanoma Cocultures as Improved Models for Nanoparticle-Mediated Delivery of RNA to Tumors.Cells. 2022 Mar 17;11(6):1026. doi: 10.3390/cells11061026. Cells. 2022. PMID: 35326474 Free PMC article.
References
LinkOut - more resources
Full Text Sources

