Clinical Value of Predicting Individual Treatment Effects for Intensive Blood Pressure Therapy
- PMID: 30857410
- PMCID: PMC6756170
- DOI: 10.1161/CIRCOUTCOMES.118.005010
Clinical Value of Predicting Individual Treatment Effects for Intensive Blood Pressure Therapy
Retraction in
-
Retraction of: Clinical Value of Predicting Individual Treatment Effects for Intensive Blood Pressure Therapy: A Machine Learning Experiment to Estimate Treatment Effects from Randomized Trial Data.Circ Cardiovasc Qual Outcomes. 2023 Oct;16(10):e000119. doi: 10.1161/HCQ.0000000000000119. Epub 2023 Sep 26. Circ Cardiovasc Qual Outcomes. 2023. PMID: 37751618 Free PMC article. No abstract available.
Abstract
Background: The absolute risk reduction (ARR) in cardiovascular events from therapy is generally assumed to be proportional to baseline risk-such that high-risk patients benefit most. Yet newer analyses have proposed using randomized trial data to develop models that estimate individual treatment effects. We tested 2 hypotheses: first, that models of individual treatment effects would reveal that benefit from intensive blood pressure therapy is proportional to baseline risk; and second, that a machine learning approach designed to predict heterogeneous treatment effects-the X-learner meta-algorithm-is equivalent to a conventional logistic regression approach.
Methods and results: We compared conventional logistic regression to the X-learner approach for prediction of 3-year cardiovascular disease event risk reduction from intensive (target systolic blood pressure <120 mm Hg) versus standard (target <140 mm Hg) blood pressure treatment, using individual participant data from the SPRINT (Systolic Blood Pressure Intervention Trial; N=9361) and ACCORD BP (Action to Control Cardiovascular Risk in Diabetes Blood Pressure; N=4733) trials. Each model incorporated 17 covariates, an indicator for treatment arm, and interaction terms between covariates and treatment. Logistic regression had lower C statistic for benefit than the X-learner (0.51 [95% CI, 0.49-0.53] versus 0.60 [95% CI, 0.58-0.63], respectively). Following the logistic regression's recommendation for individualized therapy produced restricted mean time until cardiovascular disease event of 1065.47 days (95% CI, 1061.04-1069.35), while following the X-learner's recommendation improved mean time until cardiovascular disease event to 1068.71 days (95% CI, 1065.42-1072.08). Calibration was worse for logistic regression; it over-estimated ARR attributable to intensive treatment (slope between predicted and observed ARR of 0.73 [95% CI, 0.30-1.14] versus 1.06 [95% CI, 0.74-1.32] for the X-learner, compared with the ideal of 1). Predicted ARRs using logistic regression were generally proportional to baseline pretreatment cardiovascular risk, whereas the X-learner observed-correctly-that individual treatment effects were often not proportional to baseline risk.
Conclusions: Predictions for individual treatment effects from trial data reveal that patients may experience ARRs not simply proportional to baseline cardiovascular disease risk. Machine learning methods may improve discrimination and calibration of individualized treatment effect estimates from clinical trial data.
Clinical trial registration: URL: https://www.clinicaltrials.gov . Unique identifiers: NCT01206062; NCT00000620.
Keywords: blood pressure; calibration; cardiovascular disease; machine learning; risk factors.
Figures
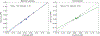
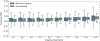
Similar articles
-
Benefit and harm of intensive blood pressure treatment: Derivation and validation of risk models using data from the SPRINT and ACCORD trials.PLoS Med. 2017 Oct 17;14(10):e1002410. doi: 10.1371/journal.pmed.1002410. eCollection 2017 Oct. PLoS Med. 2017. PMID: 29040268 Free PMC article. Clinical Trial.
-
Personalizing the Intensity of Blood Pressure Control: Modeling the Heterogeneity of Risks and Benefits From SPRINT (Systolic Blood Pressure Intervention Trial).Circ Cardiovasc Qual Outcomes. 2017 Apr;10(4):e003624. doi: 10.1161/CIRCOUTCOMES.117.003624. Circ Cardiovasc Qual Outcomes. 2017. PMID: 28373269 Free PMC article.
-
Individualising intensive systolic blood pressure reduction in hypertension using computational trial phenomaps and machine learning: a post-hoc analysis of randomised clinical trials.Lancet Digit Health. 2022 Nov;4(11):e796-e805. doi: 10.1016/S2589-7500(22)00170-4. Lancet Digit Health. 2022. PMID: 36307193 Free PMC article. Clinical Trial.
-
Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis.Lancet. 2016 Jan 30;387(10017):435-43. doi: 10.1016/S0140-6736(15)00805-3. Epub 2015 Nov 7. Lancet. 2016. PMID: 26559744 Review.
-
Treatment of Hypertension: Which Goal for Which Patient?Adv Exp Med Biol. 2017;956:117-127. doi: 10.1007/5584_2016_97. Adv Exp Med Biol. 2017. PMID: 27722961 Review.
Cited by
-
Barriers and Facilitators of Medication Adherence in Hypertension Patients: A Meta-Integration of Qualitative Research.J Patient Exp. 2024 Mar 27;11:23743735241241176. doi: 10.1177/23743735241241176. eCollection 2024. J Patient Exp. 2024. PMID: 38549805 Free PMC article.
-
Early Diagnosis of Cardiovascular Diseases in the Era of Artificial Intelligence: An In-Depth Review.Cureus. 2024 Mar 9;16(3):e55869. doi: 10.7759/cureus.55869. eCollection 2024 Mar. Cureus. 2024. PMID: 38595869 Free PMC article. Review.
-
Machine-learning-based high-benefit approach versus conventional high-risk approach in blood pressure management.Int J Epidemiol. 2023 Aug 2;52(4):1243-1256. doi: 10.1093/ije/dyad037. Int J Epidemiol. 2023. PMID: 37013846 Free PMC article.
-
Methodological concerns about "concordance-statistic for benefit" as a measure of discrimination in predicting treatment benefit.Diagn Progn Res. 2023 May 16;7(1):10. doi: 10.1186/s41512-023-00147-z. Diagn Progn Res. 2023. PMID: 37189162 Free PMC article.
-
Dynamic Importance of Genomic and Clinical Risk for Coronary Artery Disease Over the Life Course.medRxiv [Preprint]. 2023 Nov 4:2023.11.03.23298055. doi: 10.1101/2023.11.03.23298055. medRxiv. 2023. Update in: Circ Genom Precis Med. 2025 Jan 24:e004681. doi: 10.1161/CIRCGEN.124.004681 PMID: 37961553 Free PMC article. Updated. Preprint.
References
-
- Yeh RW, Secemsky EA, Kereiakes DJ, Normand S-LT, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L. Development and Validation of a Prediction Rule for Benefit and Harm of Dual Antiplatelet Therapy Beyond 1 Year After Percutaneous Coronary Intervention. JAMA. 2016;315:1735–1749. - PMC - PubMed
-
- Yeboah J, Erbel R, Delaney JC, Nance R, Guo M, Bertoni AG, Budoff M, Moebus S, Jöckel K-H, Burke GL, Wong ND, Lehmann N, Herrington DM, Möhlenkamp S, Greenland P. Development of a new diabetes risk prediction tool for incident coronary heart disease events: the Multi-Ethnic Study of Atherosclerosis and the Heinz Nixdorf Recall Study. Atherosclerosis. 2014;236:411–417. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

