Parkinson's disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration
- PMID: 30842285
- PMCID: PMC6431187
- DOI: 10.1073/pnas.1814909116
Parkinson's disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration
Erratum in
-
Correction to Supporting Information for Chen et al., Parkinson's disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2416001121. doi: 10.1073/pnas.2416001121. Epub 2024 Aug 26. Proc Natl Acad Sci U S A. 2024. PMID: 39186660 Free PMC article. No abstract available.
Abstract
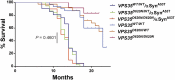
Mutations in the vacuolar protein sorting 35 ortholog (VPS35) gene represent a cause of late-onset, autosomal dominant familial Parkinson's disease (PD). A single missense mutation, D620N, is considered pathogenic based upon its segregation with disease in multiple families with PD. At present, the mechanism(s) by which familial VPS35 mutations precipitate neurodegeneration in PD are poorly understood. Here, we employ a germline D620N VPS35 knockin (KI) mouse model of PD to formally establish the age-related pathogenic effects of the D620N mutation at physiological expression levels. Our data demonstrate that a heterozygous or homozygous D620N mutation is sufficient to reproduce key neuropathological hallmarks of PD as indicated by the progressive degeneration of nigrostriatal pathway dopaminergic neurons and widespread axonal pathology. Unexpectedly, endogenous D620N VPS35 expression induces robust tau-positive somatodendritic pathology throughout the brain as indicated by abnormal hyperphosphorylated and conformation-specific tau, which may represent an important and early feature of mutant VPS35-induced neurodegeneration in PD. In contrast, we find no evidence for α-synuclein-positive neuropathology in aged VPS35 KI mice, a hallmark of Lewy body pathology in PD. D620N VPS35 expression also fails to modify the lethal neurodegenerative phenotype of human A53T-α-synuclein transgenic mice. Finally, by crossing VPS35 KI and null mice, our data demonstrate that a single D620N VPS35 allele is sufficient for survival and early maintenance of dopaminergic neurons, indicating that the D620N VPS35 protein is fully functional. Our data raise the tantalizing possibility of a pathogenic interplay between mutant VPS35 and tau for inducing neurodegeneration in PD.
Keywords: Parkinson’s disease; animal model; neurodegeneration; retromer; tau.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
VPS35 D620N knockin mice recapitulate cardinal features of Parkinson's disease.Aging Cell. 2021 May;20(5):e13347. doi: 10.1111/acel.13347. Epub 2021 Mar 21. Aging Cell. 2021. PMID: 33745227 Free PMC article.
-
VPS35 and α-Synuclein fail to interact to modulate neurodegeneration in rodent models of Parkinson's disease.Mol Neurodegener. 2023 Aug 4;18(1):51. doi: 10.1186/s13024-023-00641-4. Mol Neurodegener. 2023. PMID: 37542299 Free PMC article.
-
Parkinson's disease-linked mutations in VPS35 induce dopaminergic neurodegeneration.Hum Mol Genet. 2014 Sep 1;23(17):4621-38. doi: 10.1093/hmg/ddu178. Epub 2014 Apr 15. Hum Mol Genet. 2014. PMID: 24740878 Free PMC article.
-
VPS35 and retromer dysfunction in Parkinson's disease.Philos Trans R Soc Lond B Biol Sci. 2024 Apr 8;379(1899):20220384. doi: 10.1098/rstb.2022.0384. Epub 2024 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38368930 Free PMC article. Review.
-
Role of the VPS35 D620N mutation in Parkinson's disease.Parkinsonism Relat Disord. 2017 Mar;36:10-18. doi: 10.1016/j.parkreldis.2016.12.001. Epub 2016 Dec 5. Parkinsonism Relat Disord. 2017. PMID: 27964832 Review.
Cited by
-
De novo macrocyclic peptides for inhibiting, stabilizing, and probing the function of the retromer endosomal trafficking complex.Sci Adv. 2021 Dec 3;7(49):eabg4007. doi: 10.1126/sciadv.abg4007. Epub 2021 Dec 1. Sci Adv. 2021. PMID: 34851660 Free PMC article.
-
VPS35 D620N knockin mice recapitulate cardinal features of Parkinson's disease.Aging Cell. 2021 May;20(5):e13347. doi: 10.1111/acel.13347. Epub 2021 Mar 21. Aging Cell. 2021. PMID: 33745227 Free PMC article.
-
Vesicular Dysfunction and the Pathogenesis of Parkinson's Disease: Clues From Genetic Studies.Front Neurosci. 2020 Jan 8;13:1381. doi: 10.3389/fnins.2019.01381. eCollection 2019. Front Neurosci. 2020. PMID: 31969802 Free PMC article. Review.
-
Mitophagy, a Form of Selective Autophagy, Plays an Essential Role in Mitochondrial Dynamics of Parkinson's Disease.Cell Mol Neurobiol. 2022 Jul;42(5):1321-1339. doi: 10.1007/s10571-021-01039-w. Epub 2021 Feb 2. Cell Mol Neurobiol. 2022. PMID: 33528716 Review.
-
Aging, Parkinson's Disease, and Models: What Are the Challenges?Aging Biol. 2023;1:e20230010. doi: 10.59368/agingbio.20230010. Epub 2023 Jul 28. Aging Biol. 2023. PMID: 38978807 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

