Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells
- PMID: 30819885
- PMCID: PMC6431192
- DOI: 10.1073/pnas.1812901116
Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells
Abstract
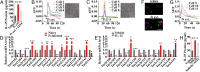
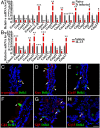
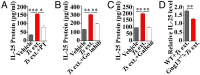
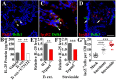
The parasitic helminth Trichinella spiralis, which poses a serious health risk to animals and humans, can be found worldwide. Recent findings indicate that a rare type of gut epithelial cell, tuft cells, can detect the helminth, triggering type 2 immune responses. However, the underlying molecular mechanisms remain to be fully understood. Here we show that both excretory-secretory products (E-S) and extract of T. spiralis can stimulate the release of the cytokine interleukin 25 (IL-25) from the mouse small intestinal villi and evoke calcium responses from tuft cells in the intestinal organoids, which can be blocked by a bitter-taste receptor inhibitor, allyl isothiocyanate. Heterologously expressed mouse Tas2r bitter-taste receptors, the expression of which is augmented during tuft-cell hyperplasia, can respond to the E-S and extract as well as to the bitter compound salicin whereas salicin in turn can induce IL-25 release from tuft cells. Furthermore, abolishment of the G-protein γ13 subunit, application of the inhibitors for G-protein αo/i, Gβγ subunits, and phospholipase Cβ2 dramatically reduces the IL-25 release. Finally, tuft cells are found to utilize the inositol triphosphate receptor type 2 (Ip3r2) to regulate cytosolic calcium and thus Trpm5 activity, while potentiation of Trpm5 by a sweet-tasting compound, stevioside, enhances tuft cell IL-25 release and hyperplasia in vivo. Taken together, T. spiralis infection activates a signaling pathway in intestinal tuft cells similar to that of taste-bud cells, but with some key differences, to initiate type 2 immunity.
Keywords: Gαo; Gβ1γ13; Ip3r2; type 2 immunity; α-gustducin.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of immune responses by tuft cells.Nat Rev Immunol. 2019 Sep;19(9):584-593. doi: 10.1038/s41577-019-0176-x. Nat Rev Immunol. 2019. PMID: 31114038 Free PMC article. Review.
-
Tuft cells utilize taste signaling molecules to respond to the pathobiont microbe Ruminococcus gnavus in the proximal colon.Front Immunol. 2023 Oct 25;14:1259521. doi: 10.3389/fimmu.2023.1259521. eCollection 2023. Front Immunol. 2023. PMID: 37954611 Free PMC article.
-
Early Trichinella spiralis and Trichinella nativa infections induce similar gene expression profiles in rat jejunal mucosa.Exp Parasitol. 2013 Oct;135(2):363-9. doi: 10.1016/j.exppara.2013.07.024. Epub 2013 Aug 8. Exp Parasitol. 2013. PMID: 23932900
-
Nitric oxide mediates intestinal pathology but not immune expulsion during Trichinella spiralis infection in mice.J Immunol. 2000 Apr 15;164(8):4229-34. doi: 10.4049/jimmunol.164.8.4229. J Immunol. 2000. PMID: 10754319
-
Induction of protection in murine experimental models against Trichinella spiralis: an up-to-date review.J Helminthol. 2015 Sep;89(5):526-39. doi: 10.1017/S0022149X15000140. Epub 2015 Mar 12. J Helminthol. 2015. PMID: 25761655 Review.
Cited by
-
Bitter taste receptors in the reproductive system: Function and therapeutic implications.J Cell Physiol. 2024 Feb;239(2):e31179. doi: 10.1002/jcp.31179. Epub 2024 Jan 14. J Cell Physiol. 2024. PMID: 38219077 Free PMC article. Review.
-
Microbiota encoded fatty-acid metabolism expands tuft cells to protect tissues homeostasis during Clostridioides difficile infection in the large intestine.bioRxiv [Preprint]. 2024 Jan 31:2024.01.29.574039. doi: 10.1101/2024.01.29.574039. bioRxiv. 2024. PMID: 38352546 Free PMC article. Preprint.
-
The Role of the Intestinal Epithelium in the "Weep and Sweep" Response during Gastro-Intestinal Helminth Infections.Animals (Basel). 2022 Jan 12;12(2):175. doi: 10.3390/ani12020175. Animals (Basel). 2022. PMID: 35049796 Free PMC article. Review.
-
Regulation of immune responses by tuft cells.Nat Rev Immunol. 2019 Sep;19(9):584-593. doi: 10.1038/s41577-019-0176-x. Nat Rev Immunol. 2019. PMID: 31114038 Free PMC article. Review.
-
Bioengineered Co-culture of organoids to recapitulate host-microbe interactions.Mater Today Bio. 2022 Jul 1;16:100345. doi: 10.1016/j.mtbio.2022.100345. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 35847376 Free PMC article. Review.
References
-
- Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–964. - PubMed
-
- Furness JB, Rivera LR, Cho HJ, Bravo DM, Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–740. - PubMed
-
- Öhman L, Törnblom H, Simrén M. Crosstalk at the mucosal border: Importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 2015;12:36–49. - PubMed
-
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

