CD20 and CD37 antibodies synergize to activate complement by Fc-mediated clustering
- PMID: 30792198
- PMCID: PMC6717598
- DOI: 10.3324/haematol.2018.207266
CD20 and CD37 antibodies synergize to activate complement by Fc-mediated clustering
Abstract
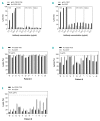
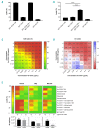
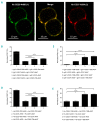
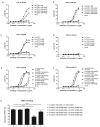
CD20 monoclonal antibody therapies have significantly improved the outlook for patients with B-cell malignancies. However, many patients acquire resistance, demonstrating the need for new and improved drugs. We previously demonstrated that the natural process of antibody hexamer formation on targeted cells allows for optimal induction of complement-dependent cytotoxicity. Complement-dependent cytotoxicity can be potentiated by introducing a single point mutation such as E430G in the IgG Fc domain that enhances intermolecular Fc-Fc interactions between cell-bound IgG molecules, thereby facilitating IgG hexamer formation. Antibodies specific for CD37, a target that is abundantly expressed on healthy and malignant B cells, are generally poor inducers of complement-dependent cytotoxicity. Here we demonstrate that introduction of the hexamerization-enhancing mutation E430G in CD37-specific antibodies facilitates highly potent complement-dependent cytotoxicity in chronic lymphocytic leukemia cells ex vivo Strikingly, we observed that combinations of hexamerization-enhanced CD20 and CD37 antibodies cooperated in C1q binding and induced superior and synergistic complement-dependent cytotoxicity in patient-derived cancer cells compared to the single agents. Furthermore, CD20 and CD37 antibodies colocalized on the cell membrane, an effect that was potentiated by the hexamerization-enhancing mutation. Moreover, upon cell surface binding, CD20 and CD37 antibodies were shown to form mixed hexameric antibody complexes consisting of both antibodies each bound to their own cognate target, so-called hetero-hexamers. These findings provide novel insights into the mechanisms of synergy in antibody-mediated complement-dependent cytotoxicity and provide a rationale to explore Fc-engineering and antibody hetero-hexamerization as a tool to enhance the cooperativity and therapeutic efficacy of antibody combinations.
Copyright© 2019 Ferrata Storti Foundation.
Figures


Comment in
-
Six-packed antibodies punch better.Haematologica. 2019 Sep;104(9):1696-1699. doi: 10.3324/haematol.2019.224196. Haematologica. 2019. PMID: 31473607 Free PMC article. No abstract available.
Similar articles
-
DuoHexaBody-CD37®, a novel biparatopic CD37 antibody with enhanced Fc-mediated hexamerization as a potential therapy for B-cell malignancies.Blood Cancer J. 2020 Apr 28;10(3):30. doi: 10.1038/s41408-020-0292-7. Blood Cancer J. 2020. PMID: 32341336 Free PMC article.
-
A novel Fc-engineered monoclonal antibody to CD37 with enhanced ADCC and high proapoptotic activity for treatment of B-cell malignancies.Blood. 2011 Oct 13;118(15):4159-68. doi: 10.1182/blood-2011-04-351932. Epub 2011 Jul 27. Blood. 2011. PMID: 21795744 Free PMC article.
-
Binding to CD20 by anti-B1 antibody or F(ab')(2) is sufficient for induction of apoptosis in B-cell lines.Cancer Immunol Immunother. 2002 Mar;51(1):15-24. doi: 10.1007/s00262-001-0247-1. Epub 2001 Dec 18. Cancer Immunol Immunother. 2002. PMID: 11845256 Free PMC article.
-
Emerging monoclonal antibodies and related agents for the treatment of chronic lymphocytic leukemia.Future Oncol. 2013 Jan;9(1):69-91. doi: 10.2217/fon.12.157. Future Oncol. 2013. PMID: 23252565 Review.
-
Molecular mechanisms of the antitumor effects of anti-CD20 antibodies.Front Biosci (Landmark Ed). 2011 Jan 1;16(1):277-306. doi: 10.2741/3688. Front Biosci (Landmark Ed). 2011. PMID: 21196171 Review.
Cited by
-
The Role of Complement in the Mechanism of Action of Therapeutic Anti-Cancer mAbs.Antibodies (Basel). 2020 Oct 28;9(4):58. doi: 10.3390/antib9040058. Antibodies (Basel). 2020. PMID: 33126570 Free PMC article. Review.
-
FcγR-Mediated Trogocytosis 2.0: Revisiting History Gives Rise to a Unifying Hypothesis.Antibodies (Basel). 2022 Jul 5;11(3):45. doi: 10.3390/antib11030045. Antibodies (Basel). 2022. PMID: 35892705 Free PMC article. Review.
-
On-target IgG hexamerisation driven by a C-terminal IgM tail-piece fusion variant confers augmented complement activation.Commun Biol. 2021 Sep 2;4(1):1031. doi: 10.1038/s42003-021-02513-3. Commun Biol. 2021. PMID: 34475514 Free PMC article.
-
Aberrant patterns of PET response during treatment for DLBCL patients with MYC gene rearrangements.Eur J Nucl Med Mol Imaging. 2022 Feb;49(3):943-952. doi: 10.1007/s00259-021-05498-7. Epub 2021 Sep 2. Eur J Nucl Med Mol Imaging. 2022. PMID: 34476551 Free PMC article.
-
Impact of structural modifications of IgG antibodies on effector functions.Front Immunol. 2024 Jan 8;14:1304365. doi: 10.3389/fimmu.2023.1304365. eCollection 2023. Front Immunol. 2024. PMID: 38259472 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

